Molecular hydrogen: a preventive and therapeutic medical gas for various diseases
Abstract
Since the 2007 discovery that molecular hydrogen (H2) has selective antioxidant properties, multiple studies have shown that H2 has beneficial effects in diverse animal models and human disease. This review discusses H2 biological effects and potential mechanisms of action in various diseases, including metabolic syndrome, organ injury, and cancer; describes effective H2 delivery approaches; and summarizes recent progress toward H2 applications in human medicine. We also discuss remaining questions in H2 therapy, and conclude with an appeal for a greater role for H2 in the prevention and treatment of human ailments that are currently major global health burdens. This review makes a case for supporting hydrogen medicine in human disease prevention and therapy.
INTRODUCTION
Oxidative stress in the cell results from the robust oxidizing potential of excess reactive oxygen species (ROS) [1]. Acute oxidative stress may result from various conditions, such as vigorous exercise, inflammation, ischemia and reperfusion (I/R) injury, surgical bleeding, and tissue transplantation [2–4]. Chronic/persistent oxidative stress is closely related to the pathogenesis of many lifestyle-related diseases, aging, and cancer [5–8]. However, many clinically tested antioxidants exhibit high toxicity levels that limit their usage to a narrow range of therapeutic dosages, and result in ineffective prevention of oxidative stress-related diseases [9]. Thus, identifying effective antioxidants with little-to-no side effects is very important for the treatment of multiple diseases.
H2 is a flammable, colorless, odorless gas that can act as a reducing agent under certain circumstances. It was previously considered physiologically inert in mammalian cells, and was not thought to react with active substrates in biological systems. Recently, H2 has emerged as a novel medical gas with potentially broad applications. Dole, et al. first reported the therapeutic effects of H2 in 1975 in a skin squamous carcinoma mouse model [10]. Thereafter, inhaling high pressure H2 was demonstrated as a treatment for liver parasite infection-induced hepatitis [11]. In 2007, Ohsawa and colleagues discovered that H2 has antioxidant properties that protect the brain against I/R injury and stroke by selectively neutralizing hydroxyl radicals (·OH) and peroxynitrite(ONOO-) [1].
To date, H2 preventive and therapeutic effects have been observed in various organs, including the brain, heart, pancreas, lung, and liver. H2 mediates oxidative stress and may exhibit anti-inflammatory and anti-apoptotic effects [12–14]. H2 not only provides a safe and effective disease treatment mechanism, but also prompts researchers to re-visit the significance and benefits of medicinal gas in the human body. This review summarizes recent progress toward potential preventive and therapeutic applications of H2 and addresses possible underlying molecular mechanisms.
POTENTIAL MECHANISMS OF H2 AS A THERAPEUTIC AGENT
The exact molecular mechanisms of the effects of low-dose H2 remain unclear. H2 can modulate signal transduction across multiple pathways, but its primary molecular targets have not been determined. Examining critical overlapping signaling molecules would help mapcrosstalk among critical pathways. To fully explain the biological functions of H2, its molecular mechanisms of action must be clarified. Potential mechanisms are proposed and summarized in Figure Figure11.
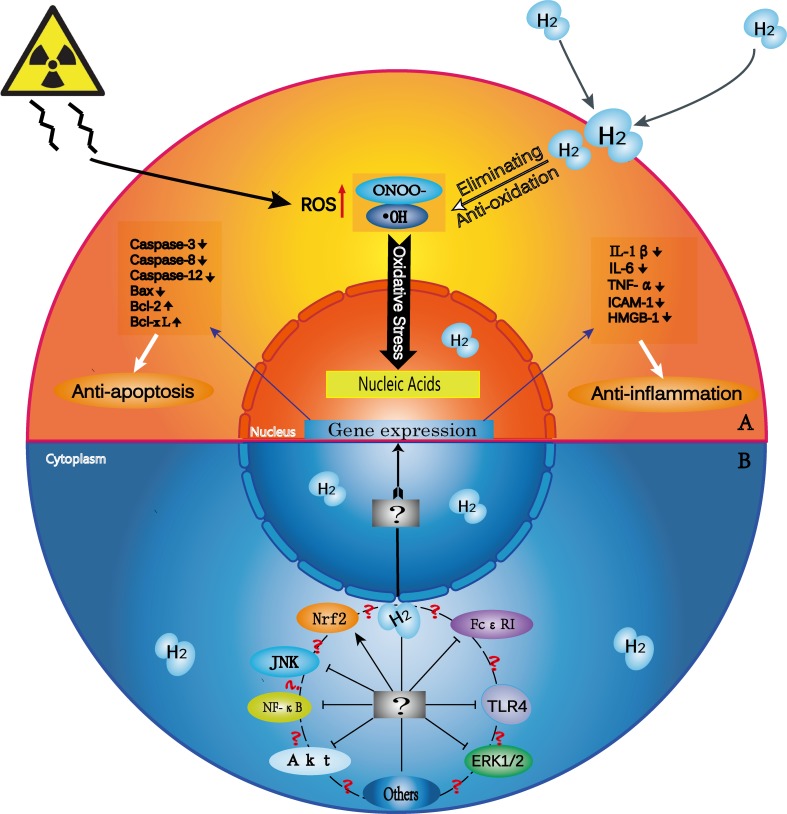
H2 biological effects and possible mechanisms of action
(A) H2 has selective anti-oxidative, anti-inflammatory and anti-apoptotic properties. Exogenous damage due to such factors as radiation induces excess cellular ROS production. H2 penetrates biomembranes and effectively reaches cell nuclei. H2 selectively scavenges ·OH and ONOO- and thus prevents DNA damage. H2 also downregulates the expression of pro-inflammatory and inflammatory cytokines, such as IL-1β, IL-6, TNF-α, ICAM-1, and HMGB-1, and of pro-apoptotic factors, such as caspase-3, caspase-12, caspase-8 and Bax. H2 upregulates the expression of anti-apoptotic factors, such as Bcl-2 and Bcl-xL. (B) H2 modulates signal transduction within and between many pathways. ?¶The exact targets and molecular mechanisms of H2 are unknown. ?: Does cross-talk occur among various signaling pathways? If so, how is it triggered? Further studies should explore other signaling pathways that may take part in H2-related disease mitigation.
Selective anti-oxidation
The role of H2 as an antioxidant has garnered the most attention among many proposed biological activities. H2 is a specific scavenger of ·OH and ONOO-, which are very strong oxidants that react indiscriminately with nucleic acids, lipids, and proteins, resulting in DNA fragmentation, lipid peroxidation, and protein inactivation. Fortunately, H2 does not appear to react with other ROS that have normal physiological functions in vivo [1].
H2 administration decreases expression of various oxidative stress markers, such as myeloperoxidase, malondialdehyde, 8-hydroxy-desoxyguanosine8-OHdG, 8-iso-prostaglandin F2a, and thiobarbituric acid reactive substances in all human diseases and rodent models [15–19]. Recent reports also revealed that H2-selective anti-oxidation mitigates certain pathological processes in plants and retains freshness in fruits [20–23]. In 2016, researchers proposed that H2 could decrease ROS content in Ganoderma lucidum depending on the presence of endogenous glutathione peroxidase [24].
Anti-inflammation
A 2001 study found that breathing high-pressure H2 could cure parasite-induced liver inflammation, and was the first demonstration of the anti-inflammatory properties of H2 [11]. H2 has exhibited anti-inflammatory activities in various injury models. Typically, H2 inhibits oxidative stress-induced inflammatory tissue injury via downregulation of pro-inflammatory and inflammatory cytokines, such as interleukin (IL)-1β, IL-6, tumor necrosis factor-α(TNF-α) [25, 26], intercellular cell adhesion molecule-1 [27], high-mobility group box 1(HMGB-1) [27], nuclear factor kappa B (NF-κB) [28], and prostaglandin E2 [29]. H2 improved survival rate and reduced organ damage inseptic mice by downregulating early and late pro-inflammatory cytokines in serum and tissues, suggesting the potential use of H2 as a therapeutic agent for conditions associated with inflammation-related sepsis/multiple organ dysfunction syndrome [30]. Additionally, H2 released from intestinal bacteria has been suggested to suppress inflammation [31].
Anti-apoptosis
H2 exerts anti-apoptotic effects by up- or downregulating apoptosis-related factors. For example, H2 inhibits expression of the pro-apoptotic factors, B-cell lymphoma-2-associated X-protein [32], caspase-3 [33], caspase-8 [32], and caspase-12 [34], and upregulates the anti-apoptotic factors, B-cell lymphoma-2 and B-cell lymphoma-extra large [32, 35]. H2 further inhibits apoptosis by regulating signal transduction within and between specific pathways. Hong, et al. first confirmed in 2014 that the H2-triggered neuroprotective effect is at least partially associated with anti-apoptotic protein kinase B pathway (also known as the Akt/glycogen synthase kinase 3β(GSK3β) pathway)activation in neurons [35].
Gene expression alterations
H2 administration induces expression of diverse genes, including NF-κB [36], c-Jun N-terminal kinase (JNK) [37, 38], proliferation cell nuclear antigen [39], vascular endothelial growth factor (VEGF) [40], glial fibrillary acidic protein (GFAP) [41, 42], and creatine kinase [43]. Some of these molecules may be secondarily regulated by H2, and some may be direct H2 targets. In the normal rat liver, H2 was found to have little effect on the expression of individual genes, but gene ontology analysis demonstrated upregulation of oxidoreduction-related genes [44]. The anti-inflammatory and anti-apoptotic properties of H2 could be realized by modulating expression of pro-inflammatory and inflammatory cytokines, and apoptosis-related factors.
H2 as a gaseous signal modulator
Oxidative stress impacts multiple signaling pathways, including the extracellular signal-regulated protein kinase (ERK)1/2, NF-κB, JNK, andnuclear factor-erythroid 2p45-related factor 2 (Nrf2) pathways. Along with selectively scavenging ·OH, H2 may alleviate oxidative stress-induced injury by targeting these pathways [45–47]. Additional studies confirmed that H2 could exert anti-inflammatory effects by regulating Toll-like receptor 4 (TLR4) signaling [48], and anti-apoptotic effects through Ras-ERK1/2-MEK1/2 and Akt pathway inactivation [49]. H2 may also protect against allergic reactions by directly modulating FcεRI-related signaling, rather than through radical-scavenging activity [50].
Since H2 may influence multiple signaling pathways to exert broad effects, crosstalk between these pathways likely influences H2 therapeutic outcomes. The effects of H2 as a gaseous signal modulator in a therapeutic setting may involve a network of signaling molecules, and future research using various animal and cell models is needed to confirm the benefits of H2 in such settings.
H2 DELIVERY MECHANISMS
Inhalation
Researchers have explored several convenient and effective delivery systems for H2 administration in vivo (Table (Table1).1). A simple method of administering H2 therapeutically is by inhalation using a ventilator circuit, facemask, or nasal cannula. Patients typically inhale H2 through a facemask, whereas in animal models, H2 is commonly administered through a ventilator that provides H2 electrolyzed from water. Inhaled H2 acts rapidly and may be used to treat acute oxidative stress [51]. An experiment in rats showed that inhalation of H2 mixed with nitrous oxide, O2, and N2 dose-dependently increased levels of H2 dissolved in arterial blood to higher concentrations than in venous blood, demonstrating that administered H2 was incorporated into tissues [1]. H2 inhalation caused no observable adverse effects and had no effects on blood pressure [1] or other blood parameters, such temperature, pH, and pO2 [52]. H2 inhalation was safe and effective in patients with acute cerebral infarction [53]. Recent findings suggest that H2 treatment is neuroprotective in patients with cerebral I/R injury [54]. H2 also mitigates surgery-induced cognitive impairment [55], decreases lung graft injury [56] and radiation-induced skin injury in rats [57], and attenuates lipopolysaccharide-induced acute lung injury in mice [14].
Table 1
| Administration | Preparation/delivery method | Characteristics |
|---|---|---|
| Inhalation | Inhale gas mixture containing H2 (< 4%) [1, 52–53] | 1. Rapid action, straightforward delivery, but unsafe. 2. Does not influence blood physiological parameters (temperature, blood pressure, pH, pO2). 3. Suitable to defense against acute oxidative stress. 4. Unpractical to dose continuously. |
| Oral intake of hydrogen-rich water (HW) | Dissolving H2 in water up to 0.8 mM under atmospheric pressure at room temperature. Drinking HW [58, 63] | 1. Convenient, easily administered, safe, efficient. 2. Easily evaporates and is lost in the stomach or intestine. 3. Difficult to control H2 concentration administered. |
| Injection of hydrogen-rich saline(HS) | Intravenous injection[122] | Delivery of more accurate H2concentrations. |
| Intraperitoneal injection[25] | ||
| Intrathecal injection [68] | ||
| Intravitreal injection [201] | ||
| Direct incorporation | Bath [69] | 1. Low cost. 2. Convenient and safe. |
| Cold storage of transplanted organs [71] | ||
| Eye drops [72] | ||
| Spray on plants or immerse plants [22] | ||
| Increased intestinal hydrogen | Oral drugs (e.g. acarbose, lactulose)[88] | 1. Low cost. 2. Convenient. |
| Dietary(e.g. turmeric) [86] |
Oral intake of hydrogen-rich water
While inhalation of H2 produces rapid effects, this delivery method may not be practical for daily preventive therapy. Due to safety concerns, H2 concentrations and dosages must be strictly controlled. Unlike gaseous H2, solubilized H2 [H2-dissolved water or hydrogen-rich water (HW)] is portable, safe, and easily administered [58]. H2 can be dissolved in water up to 0.8 mM (1.6 mg/L) under atmospheric pressure at room temperature without changing pH, and 0.8 mM HW effectively improved obesity in mice model [59]. Additionally, H2 accumulation in the liver after oral HW administration can be measured with a needle-type hydrogen electrode to determine whether consumption of small amounts of H2 over a short time period can efficiently improve various disease models. In vitro experiments demonstrated that carbohydrate polymers, including glycogen and starch, have an affinity for H2 [60], and some studies found that drinking HW produced beneficial effects in disease models, such as Parkinson’s disease [61], oral palatal wound [62], radiation-induced oxidative injuries [63], periodontal tissue aging [64], and depressive-like behavior [65].
Injection of hydrogen-rich saline
Although administering oral HW is safe and convenient, controlling the concentration of H2 administered can be difficult, as it evaporates in water over time and can be lost before absorption in the gastrointestinal tract. Thus, hydrogen-rich saline (HS) injections may deliver more accurate H2 doses [66]. Experimental evidence suggests that HS could be successfully administered by peritoneal or intravenous injection. For example, HS injection had neuroprotective effects in a spinal cord injury rat model [41]. HS treatment could also be used as an effective radioprotective agent through free radical scavenging [67], and improved survival and neurological outcome after subarachnoid hemorrhage (SAH) [25]. Additionally, intrathecal injection of HS produced analgesic effects in neuropathic rats by reducing activation of spinal astrocytes and microglia [68].
Direct diffusion of hydrogen: baths, eye drops, and immersion
Because H2 can easily penetrate the skin and be distributed via blood flow throughout the body, a warm HW bath can be used therapeutically in daily life. Warm HW baths may minimize UVA-induced skin damage [69]. A cold storage device equipped with a HW bath may be cytoprotective in various diseases and in organ transplantation. In 2011, Buchholz, et al. demonstrated that storage of intestinal grafts in a preservation solution containing high levels of H2 prevented graft damage after reperfusion [70]. In 2013, Noda, et al. found that H2 delivery to cardiac grafts during cold preservation efficiently ameliorated myocardial injury due to cold I/R. This new method for saturating organs with H2 during cold storage should be further developed for potential therapeutic and preventative use during transplantation [71].
H2 dissolved in saline has also been used to directly treat the ocular surface [72, 73]. Direct application of eye drops containing H2 ameliorated I/R injury of the retina in a rat model [72]. Antioxidant therapy via an H2-enriched irrigation solution has been suggested as a new potent corneal treatment to prevent blindness caused by alkali burn [73].
HW immersion has also drawn recent widespread attention in plant physiology. H2 was preliminarily suggested to act as a novel bioregulator involved in phytohormone signaling [74], root development [22, 75], delay of fruit senescence [23], and plant responses to various stressors, including paraquat [76], ultraviolet radiation [77, 78], drought [79], salinity [80], and cadmium [81], aluminum (Al) [21], and mercury exposure [20, 21].
Increased intestinal hydrogen
H2 is spontaneously produced in the body through fermentation of undigested carbohydrates by resident enterobacterial flora [82]. Escherichia coli can produce a considerable amount of H2 through the hydrogenase enzyme. However, few groups have studied the physiological and therapeutic functions of H2 derived from the gastrointestinal tract. H2 produced by bacterial fermentation in the gut shortens colonic transit, and this effect was more prominent in the proximal than the distal colon [83]. Kawai, et al. demonstrated that H2 released from intestinally colonized bacteria could alleviate concanavalin A-induced mouse hepatitis [31]. Endogenous H2 also mediated the suppression of colon inflammation induced by dextran sodium sulfate [84].
Recent work suggests that some oral drugs and foods stimulate intestinal H2 production, and these findings may expand the role of H2 in disease treatment. Acarbose, an α-glucosidase inhibitor, increased H2 production and neutralized oxidative stress in the gastrointestinal tract. Thus, Suzuki, et al. proposed that H2 produced by intestinal bacteria acts as a unique antioxidant and prevents cardiovascular events [85]. Dietary turmeric also induced H2 production by intestinal bacteria [86], and lactulose was shown to be an indirect antioxidant ameliorating inflammatory bowel disease [87, 88]. These examples illustrate that endogenous H2 production has important consequences in the human body.
PREVENTIVE AND THERAPEUTIC APPLICATIONS OF H2
Safety is a primary concern with respect to H2 transportation, storage, and administration. H2 is flammable only at temperatures greater than 527°C, and explodes by rapid chain reaction with oxygen in the H2 concentration range of 4–75% (vol/vol) [89, 90]. As H2 is not cytotoxic even at high concentrations, high-pressure H2 has been safely used in deep-diving gas mixes to prevent decompression sickness and arterial gas thrombi [91–93]. Because inhaling 1–4% H2 has demonstrated great efficacy in medical applications, the use of H2 at such low concentrations has been deemed feasible and safe [1, 94].
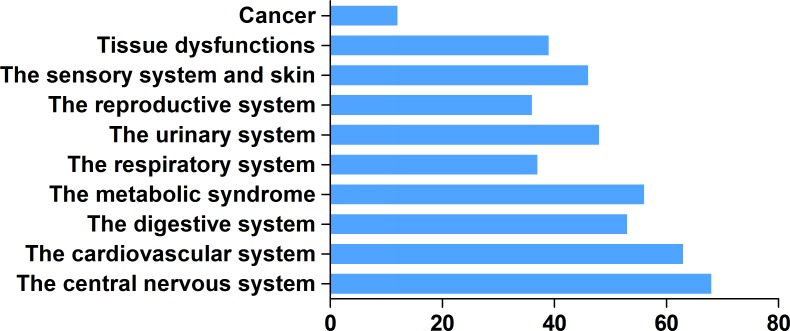
H2 has unique advantages in clinical applications. It effectively penetrates biomembranes to reach cell nuclei and mitochondria [90], and can easily penetrates the blood–brain barrier by gaseous diffusion, while most antioxidant compounds cannot. Real-time monitoring of H2 diffusion can be accomplished by measuring H2 concentrations inside various tissues using electrodes [72, 94]. As of March 2017, the number of publications on the biologically or medically beneficial effects of H2 had surpassed 450 (Figure (Figure2).2). H2 administration has shown preventive and therapeutic effects in a wide range of disease models and human diseases (Supplementary Table 1). Thus, this review will summarize the results of recent experimental and clinical examinations of actual H2 applications.
Effects of hydrogen on central nervous system diseases
Because H2 can penetrate the blood–brain barrier by gaseous diffusion [1, 95], the therapeutic effects of H2 on central nervous system diseases have been studied extensively. Ohsawa and colleagues reported in 2007 that inhaled H2 reduced infarct size in a focal cerebral I/R injury rat model [1]. Parkinson’s disease researchers found that oral HW, even at concentrations as low as 5%, alleviated symptoms in murine models by reducing oxidative stress [61, 96]. Further study indicated that drinking HW and intermittent H2 exposure were more effective than continuous H2 exposure [97]. Yoritaka, et al. recently demonstrated that drinking HW reduced oxidative stress and improved patient symptoms in a Parkinson’s disease clinical trial [98]. Moreover, endogenous H2 maybe closely related to the pathogenesis of Parkinson’s disease. Brenner, et al. found that environmental toxins deteriorated intrinsic melanin, and that melanin could split the water molecule into hydrogen and oxygen, suggesting that a lack of endogenous H2 could accelerate Parkinson’s disease processes [99]. H2 has also been studied as a potential treatment for Alzheimer’s disease, another neurodegenerative condition. Li, et al. reported that HS injection improved cognitive and memory functions in an Alzheimer’s-like rat model by preventing neuroinflammation and oxidative stress [100], likely due in part to H2-mediated suppression of abnormal IL-1β, JNK, and NF-κB activation [47].
In addition to neurodegenerative diseases, H2 administration also appears to alleviate other brain diseases and injuries, such as hypoxia-ischemia (HI) brain injury [101], stress or age-related cognitive impairment [95, 102], traumatic brain injury [103], cerebral I/R injury [104–106], and SAH-induced early brain injury [25, 107] in rodent models. However, conflicting observations have been made regarding the effects of H2 on rat brain damage. Some researchers reported beneficial effects of H2 therapy in the neonatal HI rat model [66], while others considered H2 ineffective [108]. These opposing findings might be due to differing experimental conditions, such as different degrees of HI insult, age of pups, H2 concentration, and length of H2 exposure. A recent study showed that H2 administration without surgery did not exert neuroprotective effects or improve functional outcomes in rats after intracerebral hemorrhage [109]. For spinal cord injury, H2 treatment improved locomotor behavior recovery in rats [110] and neurological recovery in mice with experimentally-induced autoimmune encephalomyelitis [111].
Effects of hydrogen on cardiovascular system diseases
Evidence suggests that H2 treatment protects against myocardial injury and development of atherosclerosis and other vascular diseases. H2 inhalation limited myocardial infarction extent without altering hemodynamic parameters in a rat model of myocardial I/R injury [94], consistent with other reports that HS injection provided cardioprotection against I/R injury [112–115]. Myocardial cold I/R injury following heart transplantation is a major determinant of primary graft dysfunction and chronic rejection [116], and can promote the subsequent development of graft coronary artery disease [117]. Researchers found that H2 inhalation ameliorated rat cardiac cold I/R injury [118], and drinking HW daily may protect cardiac and aortic allograft recipients from inflammation-associated deterioration [119]. Noda, et al. recently established a novel method of preserving cardiac grafts using a HW bath [71]. Soluble H2 delivered to excised cardiac grafts during cold preservation ameliorated cold I/R injury in grafts from syngeneic older donors and in allografts subjected to extended cold storage [71].
In addition to treating myocardial I/R injury, HS treatment prevented left ventricular hypertrophy in spontaneously hypertensive rats [120], isoproterenol-induced rat myocardial infarction [113], and doxorubicin-induced rat myocardial injury [121], and improved survival and neurological outcomes after cardiac arrest/resuscitation in rats [122]. Drinking HW alleviated radiation-induced myocardial injury in mice [123]. H2 inhalation also improved survival and functional outcomes in a post-cardiac arrest syndrome rat model [124]. In 2008, Ohsawa, et al. found that oral HW prevented atherosclerosis development in anapolipoprotein E knockout mouse model [125]. HS administration has been shown to prevent neointima formation after carotid balloon injury by suppressing ROS and the TNF-α/NF-κB pathway [126], as well as cerebral vasospasm occurrence after SAH by limiting vascular inflammation and oxidative stress in rats [127].
Effects of hydrogen on digestive system diseases
In 2001, Gharib, et al. discovered that breathing high-pressure H2was protective against parasite-induced liver injury [11]. Subsequent studies demonstrated HW therapeutic effects in concanavalin A-induced mouse hepatitis [31] and chronic hepatitis B in patients [128]. Liver fibrosis is a universal consequence of chronic liver diseases, and sustained hepatocyte injury initiates an inflammatory response. H2-mediated suppression of liver fibrogenesis in mice may be mediated mainly by ·OH scavenging, which protects hepatocytes from injury [58]. In a cirrhotic rat model, HS combined with N-acetylcysteine alleviate doxidative stress and angiogenesis [40]. H2 inhalation also reportedly protects against hepatic I/R injury [129]. Liu, et al. demonstrated that intraperitoneal injection of HS might be a widely applicable method to attenuate hepatic I/R injury in a rat model [130]. Additionally, many studies have demonstrated protective effects of H2 in other liver diseases, such as radiation-induced damage in liver tumor patients [131], acetaminophen-induced hepatotoxicity [132], obstructive jaundice-induced liver damage [45, 133], nonalcoholic steatohepatitis and hepatocarcinogenesis [134], postoperative liver failure after major hepatectomy [135], liver regeneration after partial hepatectomy [39], and acute hepatic injury in acute necrotizing pancreatitis [136] in murine models. Recent work confirmed that HS improved nonalcoholic fatty liver disease by alleviating oxidative stress and activating peroxisome proliferatoractivated receptor α (PPARα) and PPARγ expression in rat hepatocytes [137].
Intestinal I/R injury occurs in a variety of clinical settings, such as surgical treatment for abdominal aortic aneurysm, small intestinal transplantation and mesenteric artery occlusion. Inflammation and oxidative stress induced by intestinal I/R injury are the primary causes of surgical treatment [138, 139]. Injection of HS/hydrogen-rich solution reduced inflammation and oxidative stress in an I/R injury rat model, and was protective against intestinal contractile dysfunction and damage [140–142]. Poor preservation and I/R injury during small intestinal transplantation are still major causes of recipient morbidity and mortality. Buchholz, et al. demonstrated in 2008 that H2 treatment ameliorated transplant-induced intestinal injuries, including mucosal erosion and mucosal barrier breakdown, in a rat small intestinal transplant model [27]. Three years later, the same group demonstrated that intestinal grafts preloaded with H2 exhibited superior morphology and function in rodent intestinal transplants, ultimately facilitating recipient survival [70]. HS treatment also alleviated colonic mucosal damage [143] and postoperative ileus [144] in murine models.
H2 administration also has been shown to effectively treat stress-associated gastric mucosa damage [145] and aspirin-induced gastric lesions [146]. Xue, et al. found that drinking hydrogen-rich electrolyzed water suppressed the dose-response effect of aspirin-induced gastric injury in a rat model [147]. HS injection also reduced the severity of acute pancreatitis [13, 28] and I/R injury after pancreatic transplantation in rats [148].
Effects of hydrogen on metabolic syndrome
Metabolic syndrome refers to a common disorder characterized by a combination of obesity, dyslipidemia, hypertension, and insulin resistance [149]. Oxidative stress has been implicated in metabolic syndrome [150], and many studies have demonstrated protective effects of H2 in metabolic disorders [19, 151–153]. In some specific metabolic syndrome rat models, colonic H2 generated from fructan appeared to mitigate inflammation-induced oxidative stress [151]. HW also prevented glomerulosclerosis and ameliorated creatinine clearance [153]. Moreover, HS administration decreased plasma low-density lipoprotein cholesterol (LDL-C) levels and improved high-density lipoprotein (HDL) function in hamsters fed a high fat diet [154]. For patients with potential metabolic syndrome, HW consumption downregulated oxidative stress indicators and enhanced superoxide dismutase (SOD) levels, thereby increasing endogenous antioxidant defense against O2–· [19]. HW consumption also decreased patient serum LDL-C levels and improved HDL function [152].
H2 treatment has shown positive effects on energy metabolism. Kamimura, et al. found that long-term HW consumption decreased body fat and weight, along with plasma glucose, insulin, and triglyceride levels, by stimulating energy metabolism [59]. This work found that H2 treatment increased expression of the hepatic hormone, fibroblast growth factor 21, which enhances fatty acid and glucose expenditure [59].
H2 treatment also mitigates type-2 diabetes development by reducing oxidative stress and improving glucose metabolism [155]. Based on the observation that acarbose induces endogenous H2 production, Suzuki, et al. discovered that acarbose treatment increased exhaled H2 concentrations, reducing the risk of cardiovascular disease in patients with impaired glucose tolerance or type-2 diabetes. These benefits can be attributed, at least in part, to the ability of acarbose to neutralize oxidative stress by increasing H2 production in the gastrointestinal tract [85]. Amitani, et al. demonstrated that H2 could exert metabolic effects similar to those of insulin and may also be a novel therapeutic alternative to insulin in the treatment of type 1 diabetes mellitus [156].
Effects of hydrogen on respiratory system diseases
H2 treatment is beneficial in treating diverse respiratory system diseases. HS injection is protective against acute pulmonary I/R injury in rat [157] and rabbit [158] models via anti-oxidative, anti-inflammatory, and anti-apoptotic mechanisms. H2 inhalation also ameliorated lung transplant-induced I/R injury [32, 159]. Meng and colleagues recently demonstrated that inflation with CO or H2 protected against I/R injury in a rat lung transplantation model, and this effect was enhanced by combined CO and H2 treatment. H2 might exert protective effects through CO regulation, which could explain why the combination treatment exhibited greater protective effects. However, this study did not measure CO and H2 concentrations in recipient blood, and optimal CO and H2 concentrations must be further explored [160].
Recent studies have focused on H2 protection against sepsis-related lung injury. HS treatment inhibited sepsis-induced acute pulmonary injury in rats, possibly as a result of HS anti-oxidative and anti-inflammatory activities [161]. H2 inhalation also protected against sepsis-related lung injury by reducing inflammatory cytokine HMGB1 levels in septic mice, and this was partially mediated through activation of hemeoxygenase 1(HO-1) and its upstream regulator, Nrf2 [162]. In 2016, Tao, et al. demonstrated that HS administration preserved levels of aquaporin 1 (AQP1) and AQP5, which eliminate extravascular lung water, to alleviate sepsis-related lung injury by inhibiting p38 mitogen-activated protein kinase and JNK activation [37]. These observations provide potential new therapeutic targets for sepsis-related lung injury.
Studies have also shown that H2 improves lung injuries induced by many other factors, such as hyperoxia [163, 164], lipopolysaccharides [14, 17], smoke inhalation [165], paraquat[166], monocrotaline [167], and extensive burns [168]. A 2013 study showed that HS pretreatment ameliorated cigarette smoking-induced airway mucus production and airway epithelium damage in rats [169]. Xiao, et al. found that HS reduced airway inflammation and remodeling in asthmatic mice via NF-κB inactivation [46].
Effects of hydrogen on urinary system diseases
Renal I/R injury, an important cause of acute kidney injury, is unavoidable during various clinical situations, such as renal transplantation, partial nephrectomy, and treatment of suprarenal aortic aneurysms [170–172]. The mechanisms responsible for renal damage remain largely unknown, although ROS, inflammatory responses, and apoptosis are likely involved [173, 174]. Recent findings suggest that H2 protects against renal I/R injury, mainly due to H2 anti-inflammation and anti-apoptosis effects and selective reduction of cytotoxic ROS [175, 176].
Abe and colleagues associated I/R-induced acute renal injury with decreased allograft survival in patients with transplanted kidneys [177]. Allograft pre-preservation in Hydrogen-rich University of Wisconsin(HRUW) solution attenuated renal cold I/R injury caused by renal transplantation, and suppressed cytotoxic ROS generation, renal tubular injury, and interstitial fibrosis, leading to superior long-term renal graft outcomes [177]. Pre-preservation had no effect on interferon-γ, IL-6, and TNF-α expression. A 2010 study demonstrated that oral administration of HW attenuated local production of these inflammatory markers in a kidney allotransplantation setting [178]. We attribute differences in these findings to diverse H2 delivery systems and durations, and we suggest that long-term oral administration of HW appeared to have better therapeutic effects than transient pre-preservation in HRUW. Recent work indicates that HS protects against acute renal injury after liver transplantation partly by reducing apoptosis, which was possibly involved in modulating p53-mediated autophagy [33].
Various animal models have been established to study the therapeutic effects of H2 on renal injury. Nakashima-Kamimura, et al. reported in 2009 that both H2 inhalation and oral HW alleviated cisplatin-induced nephrotoxicity without compromising anti-tumor activity [60]. More recent evidence indicated that H2 alleviates renal injury induced by many factors, such as ferric nitrilotriacetate-induced nephrotoxicity [179], glucose and α, β-dicarbonyl compound-induced oxidative stress [180], unilateral ureteral obstruction [181], spontaneous hypertension [36], glycerol [43], septic shock [182], acute pancreatitis [183], and burns [184].
At present, few groups have published studies on the effects of H2 in the bladder. Matsumot, et al. found no obvious efficacy of HW in patients with interstitial cystitis/painful bladder syndrome, although supplementation with HW effectively relieved bladder pain in some cases [185]. Appropriately designed, large scale, prospective clinical studies will be required to confirm these findings.
Effects of hydrogen on reproductive system diseases
H2 has also been applied in reproductive system ailments, primarily testicular injury. The testis is highly sensitive to damage during therapeutic irradiation[186], and radiotherapy can induce azoospermia or infertility[187]. In 2012, Chuai and colleagues demonstrated that HS attenuated male germ cell loss and protected spermatogenesis with no adverse side effects in a radiation-induced mouse model [188, 189]. This represented the first in vivo evidence to suggest H2 radioprotection through ·OH neutralization in irradiated tissue. HS was also shown to play a radio-protective role in a gamma ray-induced rat testicular damage model [190]. Thus, H2therapy may effectively preserve fertility in males exposed to irradiation. Additionally, HS protects against I/R- and spinal cord hemisection-induced testicular injuries in rat models [191, 192]. Long-term HS treatment alleviated nicotine-induced testicular oxidative stress in a mouse model [193] and was protective against erectile dysfunction in a streptozotocin-induced diabetic rat model [194].
To date, only two articles have reported the therapeutic effects of H2 in female reproductive diseases. In 2011, Yang, et al. suggested that HS acts protectively in a preeclampsia rat model via effective anti-oxidation [195]. HS also attenuated chemotherapyinduced ovarian injury in a female rat model by suppressing immoderate oxidative stress, which may regulate the Nrf2/antioxidant response element signaling pathway [196]. While these investigations provide some quantitative basis for the possible use of H2 as a radio/chemotherapy-protectant, further studies are necessary to determine the exact mechanisms of action.
Effects of hydrogen on sensory system and skin diseases
Retinal I/R injury exists in various eye diseases, including glaucoma and other ocular vascular disorders [197]. In 2010, Oharazawa, et al. found that administration of H2-loaded eye drops protected the retina against acute I/R injury by scavenging ·OH, which is a highly effective neuroprotective and anti-oxidative strategy [72]. Intraperitoneal injection of HS and inhaled high-dose H2were both found to confer neuroprotection against retinal I/R injury via anti-oxidative, anti-inflammatory, and anti-apoptotic pathways in rat models [198, 199]. Unexpectedly, HS therapy did not inhibit retinal neovascularization in anoxygen-induced retinopathy mouse model [200]. Additional experiments are needed to explore the pathological and biochemical mechanisms underlying these effects.
H2 mitigated retinal diseases induced by other factors, such as glutamate-induced excitotoxic injury [201], light-induced damage [16], optic nerve crush [202], and N-methyl-N-nitrosourea (MNU)-induced retinitis pigmentosa [203]in rodent models. H2 may also be a new potent treatment for corneal injury caused by alkali burn [73], and has demonstrated protective effects in ear diseases. H2 facilitated the recovery of hair cell function and attenuated noise-induced temporary hearing loss by scavenging detrimental ROS formed in the inner ear in mouse and guinea pig models [204–208]. Another recent study suggested that HS attenuates eosinophil activation in a guinea pig model of allergic rhinitis by reducing oxidative stress [209].
The skin is a biological defense barrier for the body, and skin injuries caused directly by radiation energy or indirectly by free radicals results in radiodermatitis in nearly 95% of patients receiving radiation therapy. H2 administration protected against γ or X-ray radiation-induced dermatitis [57, 210] and ultraviolet (UV)-induced skin injury[211] in murine models. In 2013, Shin, et al. also observed that the application of atomic hydrogen surrounded by water molecules (H(H2O)m) may prevent UV-induced human skin injury [212]. H2 administration has also shown potential therapeutic effects in acute erythematous skin diseases [213], skin flap I/R injury in rats [214, 215], and psoriatic skin lesions [216]. A recent study found that autophagy played an important role in HS-attenuated post-herpeticneuralgia (PHN) in rats. Thus, HS may attenuate hyperalgesia and inhibit the release of cytokines TNF-α, IL-1β, IL-6 in rats with PHN by activating autophagy [217].
Effects of hydrogen on tissue dysfunctions
In 2011, Hanaoka, et al. demonstrated that H2 protected cultured chondrocytes against oxidative stress by selectively reducing ONOO-[218], suggesting that H2 could be used to prevent or treat joint diseases. H2 reduced disease activity in rheumatoid arthritis patients [219], alleviated microgravity-induced bone loss [220], suppressed periodontitis progression by decreasing gingival oxidative stress [209, 221–223], and prevented steroid-induced osteonecrosis in rabbits [224, 225].
H2 may also exert therapeutic effects in hematological system diseases. Allogeneic hematopoietic stem cell transplantation is a potentially curative therapy for many malignant and nonmalignant hematologic diseases. However, acute graft-versus-host disease (aGVHD) is a lethal complication of hematopoietic stem cell transplantation, which limits its application. HS administration protected against lethal aGVHD in a major histocompatibility complex-incompatible mouse bone marrow transplantation model [226] and increased survival rates in a lethal irradiation-induced mouse model [227]. Sepsis is the most common cause of death in intensive care units. Combination therapy with H2 and hyperoxia or HS treatment provides enhanced therapeutic efficacy via both anti-oxidative and anti-inflammatory mechanisms, and might be a clinically feasible approach to treat sepsis [228–231]. Other studies indicated that H2 administration accelerated recovery in aplastic anemia mice [232], increased blood alkalinity in physically active men [233, 234], inhibited collagen-induced platelet aggregation in healthy humans and rats [235], and elevated serum anti-oxidative function in thoroughbred horses [236].
Additionally, drinking HW improved mitochondrial and inflammatory myopathies in humans [237], ameliorated Duchenne muscular dystrophy in mice [238], reduced glycerol-induced rhabdomyolysis in rats [43], and alleviated muscle fatigue caused by acute exercise in athletes [239]. In 2013, Chen, et al. showed that HS attenuated fetal bovine serum-induced vascular smooth muscle cell proliferation and neointimal hyperplasia by inhibiting ROS production and inactivating Ras-ERK1/2-MEK1/2 and Akt signaling. Thus, HS may prevent human restenosis [49]. HS administration was also shown to ameliorate skeletal muscle [240] and myocardial I/R injury in rats [112, 241].
Effects of hydrogen on cancer
A growing number of studies have found that human tumor cells can produce more ROS than non-transformed cell lines, promoting cancer cell proliferation, DNA synthesis, angiogenesis, invasion, and distal metastasis [242–244]. In light of the powerful ability of H2 to scavenge free radicals, H2 administration is being increasingly studied as part of anti-cancer therapies in humans and other animals. Dole, et al. noted in 1975 that hyperbaric H2 therapy caused skin tumor regression in hairless albino mice with squamous cell carcinoma [10]. Recently, platinum nanocolloid-supplemented HW was reported to exert more rapid antioxidant activities and preferentially inhibited human tongue carcinoma cell growth as compared with normal cells [245]. Ionizing radiation can lead to carcinogenesis, and in 2011, Zhao and colleagues first reported that HS injection protected BALB/c mice against radiation-induced thymic lymphoma [246]. Other studies demonstrated that drinking HW prevented progression of nonalcoholic steatohepatitis and accompanying hepatocarcinogenesis in mice by reducing hepatic oxidative stress, inflammation, and apoptosis [134], and protected against ferric nitrilotriacetate-induced nephrotoxicity and early tumor promotional events in rats [179].
H2 can also alleviate adverse effects induced by cancer radiotherapy or anti-tumor drugs. Kang, et al. suggested that daily consumption of HW could mitigate radiotherapy-induced oxidative stress and improve quality of life after radiation exposure without compromising anti-tumor effects in patients with liver tumors [131]. Similarly, H2 administration protected against cisplatin-induced nephrotoxicity [60, 247], and doxorubicin-induced cardiac and hepatic injury [121]. These findings suggest that H2 has potential as an anti-cancer therapeutic, and could be used to reduce radio/chemotherapeutic side effects in patients.
Hydrogen in current clinical healthcare
H2 is difficult to dissolve in water, and this initially limited its therapeutic applications. In 2009, Japan solved this technical problem and produced HW. In 2012, HW sales in Japan online alone reached 20 billion yen. In the same year, researchers from 12 developed countries, including the United States and Germany, began developing H2 as a healthcare product, and the global HW market reached $22 billion. H2 industries continue to grow, and now include H2-based hydrogen-rich peripheral products, such as hydrogen health capsules, hydrogen cosmetics, hydrogen-rich bathing agents, and hydrogen ventilator equipment. The first Chinese state-owned HW brand, “Hydrovita,” was established in Beijing in 2013. The Chinese State Drug Administration subsequently defined H2 inhalation as medical behavior in 2015. The Chinese H2 market will likely be very large, since there are nearly 300 million chronic disease patients in this country. Accordingly, H2 products have a promising future as safe, simple, convenient products for health maintenance, with broad potential applications [248].
FUTURE DIRECTIONS: PROBLEMS TO BE RESOLVED
Although H2 has promising preventive and therapeutic applications in various diseases, many problems remain unresolved. Roughly 40 g of carbohydrate is thought to enter the normal human colon each day, so enormous (12,000 ml/day) quantities of H2 should be released into the colonic lumen [249–251]. The amount of intestinal H2 produced is much larger than that of H2 absorbed from water or gas, but only the effects of exogenously administered H2 have attracted the attention of the medical field at present. However, intestinal H2 also been shown to have beneficial effects in disease remission. In a mouse model, restitution of a hydrogenase-positive E. coli strain ameliorated concanavalin A-induced hepatitis [31], although drinking HW was more effective than restitution of hydrogenase-positive bacteria in this study. The fact that some exogenous oral drugs or foods stimulate intestinal H2 production supports the development of combination therapies in animal models and clinical trials. We propose that intestinal H2 therapies could expand the role of H2 in disease treatment.
No H2 dose-response effects have been observed thus far. Drinking HW reduced dopaminergic neuron loss in a mouse model of Parkinson’s disease. Notably, H2 concentrations as low as 0.08 ppm exhibited nearly the same effects as saturated HW (1.5 ppmH2) [96]. After HW is consumed, most H2 in the blood is undetectable within 30 min [178], likely due to expiration from the lungs. Thus, how a low amount of HW over a short exposure period can be effective remains unknown. However, Kamimura and colleagues found that H2 could accumulate in the liver with glycogen, which may partly explain this phenomenon [59]. In another example, as a 2% gas, the amount of H2 exposed to a 60-kg person for 24 h would be 104 or more times higher than that administered by drinking saturated HW. Nevertheless, HW is as effective as, and sometimes more effective than, H2 [252]. Therefore, the amount of administered H2 seems to be independent of the magnitude of effects in many cases.
Additionally, the molecular mechanisms and primary molecular targets of exogenously administered low-dose H2 are still unclear. Although H2 regulates the expression of various genes and protein activation states, it remains to be determined whether such modulations are the cause or result of the physiological effects of H2. Another important question is how H2 utilizes and effects crosstalk among anti-oxidative, anti-inflammatory, anti-apoptotic, and other biochemical pathways [89]. Far fewer clinical trials examining H2 applications have been conducted compared with the many animal model experiments. Nevertheless, promising applications for H2 treatment are expected to emerge for many human diseases, and personalized treatments for patients are a therapeutic goal. Thus, appropriately designed, large-scale, prospective clinical studies are warranted to optimize H2 dose, timing, and delivery methods.
CONCLUSIONS
H2 administration is a promising therapeutic option for the treatment of a variety of diseases. This article reviewed current medical research progress with respect to H2, including its unique properties, possible mechanisms of action, delivery methods, applications in animal models and clinical trials, and future applications in the field. Although important questions remain unanswered, H2-based therapies show great promise as novel and innovative tools to prevent and treat human ailments that are currently major health burdens globally. A better understanding of H2 pharmacokinetics and biological mechanisms of action will no doubt advance this important molecule in clinical applications.
Content retrieved from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5731988/.