Therapeutic potentials of superoxide dismutase
Abstract
Superoxide dismutases (SODs) constitute a very important antioxidant defense against oxidative stress in the body. The enzyme acts as a good therapeutic agent against reactive oxygen species-mediated diseases. The present review describes the therapeutic effects of SOD in various physiological and pathological conditions such as cancer, inflammatory diseases, cystic fibrosis, ischemia, aging, rheumatoid arthritis, neurodegenerative diseases, and diabetes. However, the enzyme has certain limitations in clinical applications. Therefore, SOD conjugates and mimetics have been developed to increase its therapeutic efficiency. Here, an overview is provided of some in vivo therapeutic effects observed with SOD.
Introduction
Superoxide dismutases (SODs) are a group of metalloenzymes that are found in all kingdoms of life. SODs form the front line of defense against reactive oxygen species (ROS)-mediated injury.[1] These proteins catalyze the dismutation of superoxide anion free radical (O2-) into molecular oxygen and hydrogen peroxide (H2O2) [Figure 1a] and decrease O2- level which damages the cells at excessive concentration.[2] This reaction is accompanied by alternate oxidation-reduction of metal ions present in the active site of SODs.[3,4] Based on the metal cofactors present in the active sites, SODs can be classified into four distinct groups: Copper-Zinc-SOD (Cu, Zn-SOD) [Figure 1b], Iron SOD (Fe-SOD), Manganese SOD (Mn-SOD), and Nickel SOD.[5,6] The different forms of SODs are unequally distributed throughout all biological kingdoms and are located in different subcellular compartments.
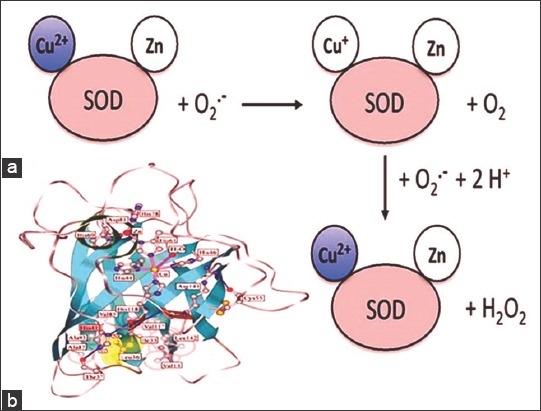
(a) The generally accepted catalytic mechanism for dismutation of O2- by superoxide dismutase (SOD). (b) Subunit structure of bovine Cu, Zn-SOD (Protein Data Bank Entry, 2 SOD)
SODs constitute a very important antioxidant defense against oxidative stress in the body.[7] Several studies have been performed that reveal the therapeutic potential and physiological importance of SOD.[8] The enzyme can serve as an anti-inflammatory agent and can also prevent precancerous cell changes.[2] Natural SOD levels in the body drop as the body ages[9] and hence as one age, one becomes more prone to oxidative stress-related diseases. SOD is used in cosmetics and personal care products as an anti-aging ingredient and antioxidant due to its ability to reduce free radical damage to the skin, therefore preventing wrinkles, fine lines, and age spots, and it also helps with wound healing, softens scar tissue, protects against UV rays, and reduces other signs of aging.[10] It has been reported that SOD has an important link in several human health problems including RBC-related disorders, cystic fibrosis (CF), postcholecystectomy pain syndrome, malignant breast disease, steroid-sensitive nephrotic syndrome, amyotrophic lateral sclerosis, neuronal apoptosis, AIDS, and cancer.[8,11-16] Furthermore, a strong association between the activity of SOD and Alzheimer’s disease has been suggested by some researchers.[8] It has also been reported that treatment with SOD helps recovery from mustard gas burns.[17] In many animal models having myocardial ischemia-reperfusion injury, inflammation, and cerebral ischemia-reperfusion injury, etc., SOD enzymes are found to be very effective.[18] SOD mimetics (small molecule catalytic antioxidants) offer a potential for treating diseases resulting from oxidative stress. SOD mimetics are synthetic compounds that mimic the native SOD by effectively converting O2- into H2O2, which is further converted into water by catalase. They are of prime interest in therapeutic treatment of oxidative stress because of their smaller size, longer half-life, and similarity in function to the native enzyme. Several attempts have been made to use SOD as a therapeutic agent against the ROS-mediated diseases. The present review describes the various therapeutic potentials of SOD.
Therapeutic Potentials of SOD
SOD and cancer
SOD, being a key cellular antioxidant, is highly responsible for the elimination of O2-. Many studies have revealed the critical role of oxidative stress in carcinogenesis.[19,20] Indeed, there are several clear evidences indicating that ROS work as endogenous class of carcinogens by inducing mutations in the cells.[21-23] Diminished activity of Cu, Zn-SOD, and Mn-SOD has been reported in cancer cells.[24,25] Normalization of SOD level contributes to part of the cancer cell phenotype reversion.[24] It has been suggested that SOD may regulate cancer progression and, hence, can be used as a novel target for cancer treatment.[26-29] Furthermore, it has been shown that Cu, Zn-SOD can be used as a novel therapeutic target for the treatment of multiple myeloma.[30] On the contrary, the invasive and migratory activity of pancreatic cancer is promoted by SOD through activation of the H2O2/ERK/NF-κB axis.[31]
SOD liposome/mimetics have experimentally shown promising results in cancer prevention animal models.[32] They have also been shown to be safe during the early phase of clinical trials. Dietary supplement-based SOD cancer prevention provides another opportunity for antioxidant-based cancer prevention.[32] SOD mimetics have been shown to be beneficial in treating the currently incurable castration-resistant prostate cancer, in which SOD-2 expression is highly suppressed.[33] It has recently been shown that a potent SOD mimetic, MnTnBuOE-2-PyP(5+), enhances carbenoxolone-mediated TRAIL-induced apoptosis in the most malignant tumor of the brain.[34]
SOD and inflammatory diseases
Neutrophils play a central and essential role in the pathogenesis of inflammation. Activated neutrophils adhere to vascular endothelium and transmigrate to the extravascular space, release ROS, protease enzymes, and large amounts of chemokines.[2] ROS and proteases damage normal tissue and extracellular matrix proteins. O2- serves to activate endothelial cells and enhance neutrophil infiltration.[35,36] Studies performed in transgenic mice overexpressing extracellular SOD[37] and SOD mimetic[36] have shown that inhibition of O2- can prevent the infiltration of neutrophils at the site of damage. Neutrophil apoptosis may also be an important step in the resolution of inflammation. In individuals with Down syndrome, neutrophil apoptosis increases and Cu, Zn-SOD is overexpressed.[38] Exogenous H2O2 together with SOD, increase the number of apoptotic neutrophils.[39]
SOD may serve as an inhibitory agent of neutrophil-mediated inflammation and may stand for a novel therapeutic approach for the ROS-dependent tissue damage induced by neutrophils through several mechanisms.[2] Preclinical studies with bovine Cu, Zn-SOD showed encouraging results for its use as a human therapeutic agent in acute and chronic inflammatory conditions, including dermatosis due to burn and wound injury.[40,41] Extracellular SOD, Mn-SOD and Cu, Zn-SOD have been described as potential inhibitor of inflammation by various reporters.[42-44]
SOD and CF
CF is characterized by the chronic inflammation and the recruitment of activated neutrophils.[45] In the plasma of patients with CF, SOD activity was significantly lower as compared with the healthy individuals.[46] Furthermore, in mononuclear, polymorphonuclear, and red cells of CF patients, reduced Cu, Zn-SOD activity was observed.[47] It has been found that the antifibrotic action of Cu, Zn-SOD is mediated by TGF-β1 repression followed by phenotypic reversion of myofibroblasts.[48] Radiation-induced fibrosis of breast was significantly reduced by Cu, Zn-SOD.[49] Proapoptotic agents induced apoptosis in CF but not in control cells that were reduced by treatment with SOD mimetic.[50] These findings indicate new therapeutic possibilities targeting antioxidant pathways including SOD, so that oxidative stress and apoptosis can be reduced in CF cells, and proinflammatory response can be limited.
SOD and ischemia
ROS including O2- and its reaction product peroxynitrite has a significant role in endothelial and tissue injury associated with ischemia and reperfusion. Overexpression of Cu, Zn-SOD reduces ischemic damage resulting from ischemia/reperfusion.[51] Mn-SOD targeted deletion deteriorates the outcome from both temporary and permanent middle cerebral artery occlusions.[52,53] The removal of O2- and peroxynitrite by SOD mimetic helps in the prevention of cellular energetic failure and tissue damage related with ischemia and perfusion and has a beneficial effect in this situation.[54]
SOD and aging
SOD is considered to be an anti-aging enzyme. The free radical theory of aging was proposed by Derham Harman.[55] It postulated that oxygen free radicals generated in metabolic pathways result in age-related deterioration through oxidative damage to biomolecules, with mitochondria being the main target of attack. Accumulation of oxidative damage is considered to be one of the key mechanisms of aging.[55-57] Drosophila flies having 75% reduction in SOD activity, showed accelerated loss of olfactory behavior on ageing.[58] It has been suggested that novel SOD mimetics may be useful in attenuating aging-induced cognitive impairments and other aspects of physiological decline with aging.[59]
SOD and rheumatoid arthritis
Rheumatoid arthritis is a systemic disease and is characterized by a chronic inflammation reaction in the synovium of joints, leading to degeneration of cartilage and erosion of juxta-articular bone. Increased oxidative stress or deficient antioxidant status is critical in the pathogenesis of rheumatoid arthritis.[60,61] Some antioxidants including SOD and Vitamin E have an anti-inflammatory role in experimentally induced arthritis.[61] It was found that SOD activity is low in patients suffering from rheumatoid arthritis and the administration of SOD through liposomes had a positive effect in the treatment of experimental arthritis.[62,63]
SOD and neurodegenerative diseases
Oxidative stress has been shown to be involved in the pathophysiology of several neurodegenerative diseases. The affected regions of patients having Alzheimer’s disease (AD) have reduced activity of antioxidant enzymes such as SOD, catalase, and glutathione peroxidase.[64,65] Familial amyotrophic lateral sclerosis (FALS) is a fatal neurodegenerative disease that leads to the selective loss of motor neurons. Several mutations in Cu, Zn-SOD gene are found to be associated with FALS.[66] In addition, Cu, Zn-SOD is one of the prime victims of oxidative damage to the brain in AD and Parkinson’s disease.[67] It has been experimentally demonstrated that overexpression of SOD-2 reduces hippocampal superoxide and hence prevents memory deficits in a mouse model of AD.[68] SOD supplementation showed improvement in mice model of AD.[69] SOD/catalase mimetic EUK-207 exhibited protection against and interruption of progression of amyloid and tau pathology and cognitive decline in a mouse model of AD.[70]
SOD and diabetes
Increased oxidative stress plays a major role in the etiology of diabetes and its complications.[71-73] In diabetes, persistent hyperglycemia stimulates the production of ROS from various sources.[74] As a result, diabetes usually leads to increased formation of ROS and weakened antioxidant defenses.[75,76] SOD catalyzes the conversion of O2- into H2O2. Under hyperglycemic conditions, endothelial cells produce elevated levels of O2-.[77] Overproduction of O2- can inhibit glyceraldehyde-3-phosphate dehydrogenase which is an important enzyme of the glycolytic pathway.[78] This leads to the accumulation of glucose and other intermediate metabolites of this pathway and shifts to other alternative pathways of glucose metabolism along with increased production of advanced glycation end products.
Treatment with SOD has experimentally been shown to reduce liver oxidative stress in diabetic animals.[79] SOD mimetic (Mn[II][pyane] Cl2) has successfully been used to treat diabetes in diabetic rats.[80] Chemically modified SOD (carboxymethylcellulose-SOD and poly methyl vinyl ether-co-maleic anhydride-SOD) was effective in treating diabetes and offers a therapeutic advantage in clinical use.[81] It has been demonstrated that extracellular SOD can act as a therapeutic agent to protect the progression of diabetic nephropathy.[82]
Current limitations of SODs for therapeutic applications
Due to the instability, high immunogenicity, low cellular uptake, and lesser circulation in vivo half-life of SOD, their clinical applications as therapeutic agents are very limited. For this reason, a wide variety of SOD conjugates have been developed with longer circulation half-lives, high stability, and lesser immunogenicity.[81,83,84] These SOD conjugates have exhibited marked effects in vivo. The administration of SOD in free form has some disadvantages, most importantly, the low accumulation of SOD in inflamed areas due to its reduced half-life in the bloodstream and its rapid renal excretion. To overcome this, SOD can be incorporated either in highly loaded conventional liposomes or long-circulating liposomes (PEG-liposomes).[85] Many SOD mimetics have been synthesized that can be used as pharmaceutical agents in a large number of diseases in which the native SOD is ineffective.[80,86] Potent SOD mimetics such as metalloporphyrins, Mn cyclic polyamines, Mn-salen derivatives, and nitroxides have been developed for treating various diseases resulting from increased oxidative stress.[87]
Future perspectives
Diets high in vegetables and fruits, which are good sources of antioxidants, have been found to be healthy. Traditional antioxidants such as selenium, carotenoids, and Vitamins E and C are safer products. However, research has not shown these antioxidant supplements to be beneficial in preventing diseases.[88,89] The reasons may be: The effects of the large doses of antioxidants used in supplementation studies may be different from those of the smaller amounts of antioxidants consumed in foods. Differences in the chemical composition of antioxidants in foods versus those in supplements may influence their effects. For some diseases, specific antioxidants might be more effective than the ones that have been tested.
Current research reveals the potential therapeutic applications of SOD in the prevention/control of various diseases. Future approaches in this field are expected to include gene therapy to produce more antioxidants in the body, increasing the level of antioxidants in plant products by genetic modifications, synthetic antioxidant enzymes (SOD mimetics). Among the most critical antioxidants that ameliorate the effects of oxidative stress within cells are enzymes such as the SODs. Due to their importance, the SODs have received much attention in efforts to minimize oxygen radical-induced damage to normal tissues. Since the administration of exogenous SODs themselves has often proven to be problematic, a variety of innovative approaches are currently being explored in conjunction with radiotherapy. Among these are SOD mimetics, the future holds great promise for the development of superior products that will serve to ameliorate the damaging effects of radiation on normal cells and tissues.
Conclusion
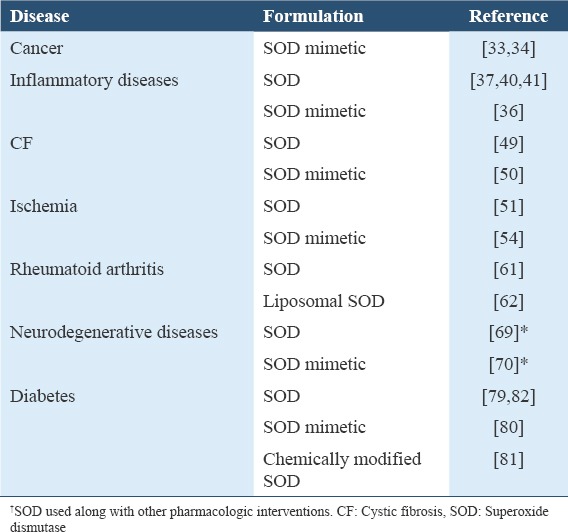
SOD can be used as a pharmaceutical in treating various diseases resulting from oxidative stress [Table 1]. SOD conjugates and mimetics have improved performance and overcome some of the limitations of the free enzyme. Antioxidant-based mimetics may potentially be the future of oxidative stress targeted therapies in chemoprevention. It is important that future research on the potential use of SOD or its conjugates and mimetics in the treatment of oxidative stress-related diseases should focus on patient-oriented outcomes.
Table 1
Some diseases in which SOD has been shown to exhibit significant therapeutic action/potential in humans/animals
Content retrieved from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5969776/.