Protein Restriction, Epigenetic Diet, Intermittent Fasting as New Approaches for Preventing Age-associated Diseases
Data from epidemiological and experimental studies have shown that diet and eating patterns have a major role in the pathogenesis of many age-associated diseases. Since 1935, calorie restriction (CR) has been identified as one of the most effective nongenetic dietary interventions that can increase lifespan. It involves reducing calorie intake by about 20%–40% below ad libitum, without malnutrition. Restricting food intake has been observed to increase lifespan and prevent many age-associated diseases in rats, mice, and many other species. Understanding the metabolic, molecular, and cellular mechanisms involved in the anti-aging effects of CR can help us to find dietary interventions that can mimic its effects. Recently, different studies have shown that intermittent fasting, protein restriction, and an epigenetic diet can have similar effects to those of CR. These approaches were selected because it has been indicated that they act through a similar molecular pathway and also, are safe and effective in delaying or preventing diseases. In this review, we focus on the mechanistic pathway involved in CR. Then, we review the mimicking interventions through the mechanistic approach. For this purpose, we reviewed both animal and human articles, mainly available through the PubMed online database. We then selected the most relevant full texts which are summarized in this article.
Introduction
Nowadays, obesity is one of the concerning issues that societies worldwide are confronting. Obesity is known as a complex interplay between genetic and environmental factors, occasionally tailored by morbidity and mortality. Being overweight and obese may be the origins or triggers of a great number of health problems, independently and in association with another disease. Food consumption has two extremes: (i) Low enough to cause death from starvation and (ii) high enough to lead to obesity. Calorie restriction (CR) is positioned between these two extremes. A CR regimen is generally carried out through the reduction of calorie intake by up to 20%–40% of ad libitum (AL), whereas adequate nutrient intake is maintained.[1,2]
In 1917, for the first time, Osborne et al.[3] reported that food intake restriction can slow growth and increase life longevity. However, due to methodological defects, it did not attract much attention. After this, in 1935, MacCay[4] published a paper which showed CR without malnutrition in rats can increase the mean and maximum length of life.[5] Many investigators worldwide have confirmed this observation and showed that CR is the most efficient nongenetic strategy for life extension in other model organisms, including yeasts, fruit flies, fish, and monkeys. An increase in the lifespan of rats has been observed when nutrient availability drops between 30% and 75% of the species’ normal calorie intake. Not only did calorie-restricted rodents live longer than the AL fed counterparts but also significant part of them (about 30%) died without any apparent pathology, raising the striking possibility that aging is not necessarily tightly linked with costly pathologies.[6,7,8,9]
On the other hand, history has many examples showing that CR, due to food deprivation, has worked to the advantage of human health. World War II reduced the food intake of many people living in Europe, leading to anti-aging benefits. Thereby, fewer cases of heart disease, hypertension, and diabetes were reported.[1] Furthermore, the incidence of cancer was lower than expected among Norwegian women who went through puberty during the food shortages of World War II.[10]
Some studies have shown the anti-cancer effects of CR; however, they are not conclusive.[11,12,13] The suggested mechanism is that long-term exposure to CR can lead to a reduction of circulating levels of several cytokines, growth factors, and hormones, accompanied by a decrease of growth factor signaling, minute vascular perturbations, and inflammation. Concomitantly, these changes caused by CR result in decreased cancer risk and progression.[14] Specifically, reduction of body weight by 20% or more, serum insulin-like growth factor 1 (IGF-1) by up to 75%, and glucose by up to 70% have been reported in mice after short-term fasting. Under these circumstances, animals similar to yeast become highly stress-resistant. Furthermore, reducing the level of IGF-1 in normal cells and mice enhances their resistance to chemotherapy-dependent damage while simultaneously sensitizing a large number of tumors to chemo and radiotherapy.[15,16] Clinical trials in morbidly obese patients exhibited that after 1 week of a very low–calorie diet (VLCD) of 400 kcal/day or 3 weeks of 500 kcal/day, insulin secretion improved.[17]
Mice homozygous knockout for the apolipoprotein E gene (ApoE−/−) under a calorie-restricted regimen (60% of total calorie) had fewer formations of atherosclerotic lesions in relatively early stages compared to the AL group.[18] Endothelial dysfunction and vascular oxidative stress due to obesity have been reversed by CR in C57Bl/6 mice.[19] Findings across the study of human and nonhuman primates indicated that CR can decrease triglycerides, blood pressure, and increase high-density lipoprotein levels.[20] CR, similar to cyclophosphamide, can delay the onset of autoimmune diseases through decreasing the proportion of B cells and preserving high numbers of native T cells and their immune responsiveness.[21]
Evidence from clinical and basic researches points to a deep connection between brain function decline and metabolic dysregulation during senescence. Excess nutrient availability may be detrimental to brain function. Conversely, a 30% reduction in calorie intake for 3 months was found to improve memory performance in elderly individuals. Aside from these observations, the results of three on-going CR studies on rhesus monkeys until now have demonstrated that aging CR-treated monkeys suffer less severe brain atrophy (hallmark of an aging brain) compared to the control fed AL.[22] In addition, it has been shown that CR can lead to up-regulation of brain-derived neurotrophic factor (BDNF) that is associated with neuronal plasticity and neurogenesis.[23]
Despite the beneficial and profitable effects of CR, Giller et al. reported that after a 6-month dietary restriction, a refeeding period abolishes approximately all the positive changes gained from the restriction period.[24] On the other hand, a 6-month dietary restriction decreased major urinary protein 5 (Mup5) in male C57BL6 mice that is responsible for communication and sex function. After this, during the refeeding period the declining flow of Mup5 was inverted.[25]
Methods
Data are based on the result of original and review articles relating to CR, the involved mechanism which it acts through and the interventions that are able to mimic CR-like effects. For this purpose, we mainly used the online database of PubMed. The following keywords were searched: CR, mechanism, protein restriction, intermittent fasting (IF), and epigenetic diet. Then, we selected the most relevant full texts and reviewed the articles. Our review includes both animal and human studies.
Underlying Mechanism
How and in which way does CR work? The answer to this question can help us find other methods and dietary patterns with similar effects. Now it is clear that the level of oxidative stress is higher in old animals compared to young ones. More so, CR feeding has the capacity to hinder the accrual of oxidative damage markers, like oxidative damage to bases in genomic and mitochondrial DNA and also, tissue concentration of protein carbonyls and peroxidized lipids. Membrane fluidity and the physical properties of lipid micro-domains can be affected by this by-product, thereby distorting cellular membrane functionality. The preservation of membrane and its fluidity by CR feeding is associated with the attenuation of age-related changes in membrane receptor numbers and binding affinity.[26] Experimental and epidemiological studies demonstrated that IGF-1 and its binding proteins have some role in the biology of aging and pathogenesis of some common cancers.[27] IGF-1 plays a key role in the differentiation and proliferation of cells and also prevents cell apoptosis of normal and cancer cells. CR can protect us against cancer and slow the aging process through decreasing IGF-1 level by about 40%.[28] Restraining insulin/IGF-1 signaling is the most effective and efficient intervention applicable to extend lifespan and prevent age-related diseases.[29]
The other theory involves the positive effect of CR on suppressing target of rapamycin (TOR) and Akt. Medvedik et al. reported that TOR inhibition extends lifespan by the same mechanism as CR, meaning it can stabilize rDNA locus and increase Sir2p activity.[30,31] The mammalian TOR (mTOR) pathway has been implicated in mammary tumor development. Therefore, removing its subunit is associated with reducing the incidence of age-related diseases such as immune and motor dysfunction, bone disease, and insulin sensitivity.[32,33]
Together, adaptations that occur due to CR are a down-regulation of the insulin/IGF pathway (i.e., PI3K/Akt/mTOR), and an upregulation of two energy-sensing pathways (i.e., sirtuin [SIRT] and activated protein kinase [AMPK]) which activate forkhead box O (FOXO). FOXO is tailored by upregulation of autophagy genes, the DNA repair gene, and down-regulation of genes that control cell proliferation.[34] Unlike the effects of FOXO on apoptosis promotion, they transactivate reactive oxygen species (ROS)-detoxifying enzymes such as catalase and superoxide dismutase 2 (SOD2/MnSOD). Hence, intracellular oxidative stress will reduce resulting in cell survival.[35,36] Therefore, the role of FOXO in apoptosis and cell survival is like a double-edged sword.
At low energetic cellular levels, AMPK is activated which is accompanied by the down-regulation of mTOR.[37] AMPK has a major role in lipid metabolism and mitochondrial biogenesis. PGC-1α, a transcriptional regulator that coordinates mitochondrial biogenesis, should be low acetylated by Nicotinamide-Adenine dinucleotid (NAD+) dependent deacetylase SIRT1 to work properly. However, its residue should be phosphorylated by AMPK for it to be recognized and interact with SIRT1. Besides AMPK increases the intracellular NAD+ level and improves SIRT1 activity.[38]
The other mechanism that plays a role in CR-mediated anti-aging benefits is autophagy. Oxidative damage to macromolecules and organelles occurs through normal metabolism. If these damaged molecules are not removed by autophagy, they change to a source for the production of free radicals that leads to oxidative stress, inflammation, and severe diseases. Autophagy functions as a protective mechanism that removes damaged or aged organelles to protect cells from further oxidative stress, dysfunction, and cell death. Data suggest that a decrease of amino acids due to CR stimulates autophagy and lysosomal proteolysis activity.[39] Moreover, studies carried out on diverse eukaryotic species show that CR is the most potent inducer of autophagy and can prevent age-associated diseases.[40] TOR inhibition and AMPK activation, resulting from CR, can activate the autophagy-promoting Unc-51 like autophagy activating kinase 1 complex in parallel with the acetyltransferase Mec-17 which stimulate microtuble transport machinery, necessary for autophagy. Furthermore, autophagic proteins will be activated through deacetylation by SIRT1 which is itself activated by CR.[41]
It has also been shown that CR reduces ROS production by down-regulating NADPH oxidase. On the other hand, it is evident that nuclear factor-erythroid 2 (Nrf2) plays a key role in vasoprotection and regulating the aging process by orchestrating the transcriptional response of cells to oxidative stress. CR restores Nrf2 expression and activity in aged cerebro microvascular endothelial cells.[42] Moreover, CR attenuates telomere erosion associated with aging and decreases cancer incidence through overexpression of telomerase.[43] The nuclear factor kappa B (NFκ-B) is also a redox-sensitive transcription factor that induces the expression of genes involved in cellular proliferation and inflammation. It has been suggested that CR increases cytoplasmic levels of IkkB which prevent NFκ-B translocation into the nucleus.[44]
CR induces the endothelial nitric oxide synthase and results in an increase in mitochondrial biogenesis. Similarly, NOS activates the SIRT1 gene in vivo and in vitro. Mammals contain seven homologs of yeast Sir2, SIRT1–7 that have numerous salutary and anti-aging effects.[45] Among them, SIRT1 has been studied most extensively. It has the ability to link the metabolic status to transcriptional outputs, thus playing a key role in energy hemostasis. It can regulate stress glucose-stimulated insulin secretion in pancreatic β-cell and promote the survival of pancreatic β-cells during oxidative stress and gluconeogenesis.[45] SIRTs have a dozen substrates, among which are age regulators, such as the FOXO family of transcription factors.[46] The effects of SIRT1 on the FOXOs’ functions are complex and highly dependent on the FOXO target genes. SIRT1 decreases the transcription of genes involved in apoptosis while promoting the expression of FOXO target genes involved in stress resistance.[36] Moreover, it is indicated that CR induces an increase in SIRT3 expression. Activation of SIRT3 during CR reduces oxidative stress by activating the mitochondrial antioxidant enzyme, SOD2 [Figure 1].[47]
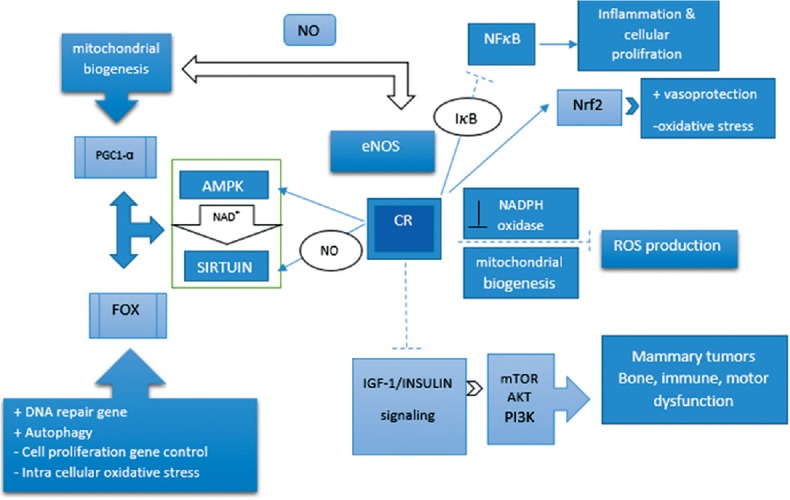
Mechanism pathway involved in calorie restriction effect. Calorie restriction upregulates activated protein kinase and sirtuin pathways and downregulates insulin-like growth factor 1/insulin pathway. Generally, calorie restriction activates a pathway that reduces inflammation, reactive oxygen species production, and oxidative stress by improving mitochondrial biogenesis and detoxifying enzyme activity. Moreover, it can regulate cell proliferation and differentiation by regulating nuclear factors: induce: —>, inhibit: —|
During aging, chromatin integrity and hemostasis capability will diminish due to aberrant gene expression. DNA methylation plays a major role in the maintenance of DNA stability and integrity. In addition, it can regulate gene expression in a variety of biological processes. Two major changes in DNA methylation occur during aging progression. These changes lead to globally decreased, but locally increased DNA methylation statuses. Interestingly, CR has the ability to correct this aging-induced improper DNA methylation pattern. Histone acetylation and deacetylation processes are catalyzed by specific enzymes called histone acetyltransferases and histone deacetylase (HDACs), respectively. In general, the more acetylated the histone amino tails become, the more likely it is that the gene promoter region containing the histones will have increased transcriptional activity. The HDACs increased activity can lead to tumorigenesis through effects on epigenetic gene expression, making HDACs over-expression a very common feature of cancer cells. It has been reported that HDAC activity increases during CR, suggesting that global deacetylation may have a protective role against nutrition stress and may impact the aging processes.[48,49]
Intermittent Fasting
The behavior and physiology of an organism are all in accordance with 24-h light/dark (LD) cycles which are controlled by evolutionarily conserved natural circadian oscillators. In mammals, LD cycles’ signals stimulate and control the central circadian clock which resides in the suprachiasmatic nucleus of the hypothalamus that eventually results in environmental adaptation.[50]
It has been suggested that increased activity during rest time in the premodern world, coupled with sleep disruption, is associated with an increased the prevalence of cardiovascular diseases, diabetes, and obesity, alongside certain cancers and inflammatory disorders.[51] On the other hand, Puttonen et al. reported that 2- and 3-night work shifts are accompanied with increased systemic inflammation.[52]
Investigators compared time-restricted feeding (tRF) to AL access, both in a high-fat diet (HFD) in mice. They have seen that “mice under tRF consume equivalent calories from HFD as those with AL access. However, the former is protected against obesity, hyperinsulinemia, hepatic steatosis, and inflammation and also, has improved motor coordination.” Hence, they have concluded that a tRF regimen improved CREB, mTOR, and AMPK pathway functions and oscillations of the circadian clock.[53] Moreover, Zarrinpar et al. have shown that gut microbiota dampened during HFDs in rats can be restored by tRF. Since gut microbiota influence the host’s metabolism, it is an important strategy against obesity and other diseases.[54]
IF decreased proinflammatory proteins such as NLRP1 and NLRP3, NF-κB, IL-1 β, and IL-18 in the brain and periphery after cerebral ischemia in mice.[55] IF combined with a ketogenic diet (KD) in children with an incomplete response to the diet had modest or transient improvements in seizure control in four of six children in the study and was attributed to the neuroprotective effects of IF.[56] Furthermore, it has been reported that IF ameliorates cognitive deficits in a rat model of sepsis by a mechanism involving suppression of pro-inflammatory cytokines, activating NFκB and enhancement of neurotrophic support. In addition, it decreases lipopolysacharid-induced elevation of interleukin (IL)-1α, IL-1β and tumor necrosis factor alpha levels, and prevents the lipopolysaccharide-induced reduction of (BDNF) levels in the hippocampus.[57] Studies of rats and mice have shown that running wheel exercise and IF increase BDNF expression in several regions of the brain and improve synaptic plasticity and neurogenesis.
In both mice and humans, fasting for 2 or 5 days, respectively, causes 30% or more decrease in glucose, over 50% decrease in IGF-I, and a 5–10-fold increase in the IGF-1 binding protein.[58] IF in rats reduces heart rate, blood pressure, and insulin levels similar to or greater than those obtained with regular physical exercise, through a mechanism involving stress responses (increasing plasma adrenocorticotropin and corticosterone). Since insulin-like signaling pathways down-regulate cellular response to stress, this is another reason for their opposite actions.[59] In the study of the relationship between eating frequency and inflammatory biomarkers, it was reported that women who ate <30% of their total daily calories in the evening and had a longer nighttime fasting duration were associated with an 8 percent lower C-reactive protein (P = 0.01).[60]
Reduction of body weight by 7% (6 kg), fat mass by 13% (4 kg,), and waist circumference (an indirect indicator of visceral fat) by 6% (6 cm) has been observed in obese women who underwent 2 days of VLCD (600 kcal/day) and ate AL on every other day of the week for 24 weeks.[61] Whereas weight changes offer an indirect assessment of the effect of IF, in the meta-analysis performed by Patterson et al., statistically significant weight reduction was observed in 85% of trials who were included in the study.[62]
Protein Restriction
The impact of CR on the lifespan of nonhuman primates is still controversial and may be influenced by dietary composition. It is believed that lifespan extension associated with CR operates through the reduction of IGF-1, insulin level and their related signaling.[63] Data from experimental and epidemiological studies indicate that protein intake is one of the most robust dietary regulators of circulating levels of IGF-1, a powerful growth factor, which activates the Akt/mTOR pathway.[64]
The study of 6,381 adults aged 50 and over from NHANES IIII showed that during 18 years of following those who reported high protein intake (>20% calorie from protein vs. <10%) there was a 4-fold increase in cancer death risk and 75% increase in overall mortality.[63] In the evaluation of CR effects on glioma progression, CR significantly reduced both IGF-1 and glucose levels, but only the complete lack of proteins reduced IGF-1 level when specific macronutrient deficiencies were tested. Mice in the group fed with a calorie-restricted high-protein diet had the worst survival of all CR groups, compared to the control group.[10] Elsewhere investigators reported that protein restriction (PR) (7% compared to 21%) inhibits tumor growth in human xenograft prostate and breast cancer models through epigenetic modification and inhibition of the IGF/Akt/mTOR pathway.[64] Moreover, protein restriction cycles were associated with an 8-fold increase in IGF factor-binding protein-1 and 30%–70% reduction in the circulating level of IGF-1. These changes accompanied with tau phosphorylation in the hippocampus and they alleviated age-dependent impairment in cognitive performance.[65]
In 1992 Yongman showed that either protein or CR markedly inhibited the accumulation of oxidatively damaged proteins. Furthermore, protein restriction (5%–10%) compared to normal protein intake (20%) reduced the accumulation of oxidatively damaged proteins during the oxidative stress of chronic irradiation.[66] Removal of the single essential amino acid tryptophan or total protein restriction reduced inflammation, resulting in protection against renal and hepatic ischemic injury and preserved organ function.[67]
Under amino acid scarcity, tRNAs convert to uncharged form, leading to the activation of general control nonderepressible 2; a protein kinase and important regulator of the general AA control pathway, resulting in slow growth. Under oxidative stress, AAs serine (Ser), threonine (Thr), and valine (Val) were the only three that caused a strong sensitization of cells to oxidative stress, and lack of these AAs was sufficient to decrease age-dependent DNA damage, thus extending lifespan.[68] Extending lifespan in both fruit flies and yeasts has been reported after methionine restriction through Tor signaling, and this effect requires low amino acid status.[69] CR, as well as a 40 and 80% protein restriction, could decrease restriction of S-(carboxymethyl)-cysteine, a mitochondria damage biomarker.[70]
Fibroblast growth factor 21 (FGF21) is implicated in the adaptive response to both starvation and KDs, and interventions that raise circulating FGF21 levels increase insulin sensitivity and energy expenditure. Laeger et al. showed that hepatic FGF21 expression is induced by dietary protein restriction (50% control).[71] Moreover, improved insulin sensitivity on short-term PR required mTOR complex 1 repressor tuberous sclerosis complex in vivo.[72]
Epigenetic Diet
Heritable changes in chromatin organization and gene expression that are not due to alteration of the DNA sequence are called epigenetics. Dietary phytochemicals such as genistein, sulforaphane (SFN), tea polyphenols, curcumin, resveratrol, vitamins, and minerals have been demonstrated to act through an epigenetic mechanism that affects the epigenome and represents another nutritional alternative to CR.[73,74] The aforementioned theory is more commonly referred to as “epigenetic diet” which introduces bioactive compounds that aid in delaying the age-associated disease processes and the onset of aging.[75]
It seems that SIRT activation is an important mechanism that polyphenols act through in order to exhibit CR-like behavior. Further evidence exists supporting a role of polyphenols in gene regulation through transcription factors such as Nrf2, forkhead box O (FOXO), NFκ-B and the gamma coactivator 1-alpha (PGC-1α).[44] Among polyphenols, Resveratrol (a polyphenol found in red wine, grape, nuts, and others) received much attention due to its anti-aging effects and mimicking CR. It is suggested that Resveratrol acts as a SIRT1 activator.[75] Rat experimental models comparing a low dose of resveratrol with AL and CR diet, showed that resveratrol can inhibit gene expression profiles associated with skeletal and muscle aging and prevent age-related cardiac dysfunction similar to CR.[76] Prostate cancer shows reduced growth and metastases when exposed to resveratrol through miR-21’s inhibition of Akt; in addition, miR-21’s interaction with NFK-B is noted in glioma cells with the same benefits.[77] Similar to resveratrol and quercetin, curcumin has also been shown to induce Nrf2 and other transcription factors and inhibit NFκ-B-mediated inflammation.[44] In vitro cell culture studies performed using B16 and S91 melanoma cells showed that SFN down-regulates the deacetylation enzyme and inhibits growth and proliferation of cancer cells.[78] Bmi-1, a PRC1 protein, is overexpressed in some human cancers, including colorectal cancer, human non-small cell lung cancer, and epidermal squamous cell carcinoma (SCC) cells. It is reported that (2)-epigallocatechin-3-gallate treatment of SCC-13 cells (a human epidermal squamous carcinoma cell line) reduced Bmi-1 and in addition, EZH2 levels were associated with reduced cell survival. These all occur through epigenetic mechanisms.[79] In vivo experiments show that male rats generate peroxides 2-fold when compared to females. However, this is changed through ovarectomy and can be reversed by estrogen therapy.[80] It is indicated that the pathway through which genistein acts to increase antioxidant capacity in cells is through “interaction with estrogen receptor (s), activation of MAP kinase, activation and nuclear translocation of NFkB, over-expression of MnSOD and lowering of the intracellular levels of oxidants.”[81]
Homocysteine is a complex biochemical pathway that is also known as one-carbon metabolism pathway. The one-carbon metabolism can be regulated by various nutrient, acting both as co-enzymes (e.g., Vitamins B2, B6, B12, and folate) and as sources of methyl donors (e.g., methionine, choline, betaine, and serine). A number of studies confirm that a folate-poor diet is associated with global DNA hypomethylation and an increased risk for chronic diseases. On the other hand, high levels of it are correlated with aging and cancer. Moreover, deficiencies in Vitamin B12 can lead to elevated DNA damage and altered DNA methylation and an increase of homocysteine levels.[82] Global hepatic DNA hypomethylation in rats in response to dietary all-trans retinoic acid has been observed, but not in response to retinyl-palmitate or 13-cis-retinoic acid.[83]
Furthermore, data from a meta-analysis suggests that supplementation with a high dose of Vitamin E may increase all-cause mortality in men and women living in developed countries.[84] A meta-analysis of total mortality in randomized trials testing Vitamin D supplementation concluded that supplementation at ordinary doses was associated with reduced total mortality rate; however, other studies in this regard are recommended.[85]
Conclusions
For a long time, calorie-restricted nutrient-rich dietary regimens have been identified as the most robust dietary intervention that can promote lifespan and prevent age-related disease. Essential pathways have been identified. Regulating IGF1, AMPK, and sirtuins pathways plays an evolutionary role in the mentioned mechanisms. The feasibility of developing CR in different people and nations raises the question of what other interventions could promote the same effects. After CR, the most promising strategies capable of having similar effects and reducing aging biomarkers are IF, protein restriction, and an epigenetic diet. Frequency and the meal’s circadian time result in a metabolic shift of nutrient metabolism and stimulation of stress response which can correct molecular damage by the same mechanism of CR. Since protein intake is the chief regulator of the IGF1 pathway, protein restriction which reduces its level has been associated with protection from many chronic diseases. Finally, dietary compounds which are safe and effective in extending lifespan, known as an epigenetic diet, can modify growth factors and sirtuins in the same way that CR works.
Content retrieved from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6036773/.