Epigenetic Modifications as Outcomes of Exercise Interventions Related to Specific Metabolic Alterations: A Systematic Review
Introduction
The prevalence of non-communicable chronic diseases is rising in all populations [1]. Several factors related to lifestyle have been described to increase the risk of these diseases, including tobacco and alcohol consumption, physical inactivity, and diets rich in saturated fats, salt, and sugars. However, other risk factors related to genetics, metabolism, the endocrine system, gut microbiota, and epigenetics also influence the susceptibility to develop chronic diseases such as obesity, dyslipidemias, insulin resistance, type 2 diabetes mellitus (T2D), cancer, and cardiovascular diseases [1, 2, 3]. These chronic diseases have been estimated to cause around 41 million deaths every year and represent 71% of all deaths worldwide [4, 5].
In recent years, epigenetics has gained more attention since it has been shown that environmental factors, including diet and physical exercise, may alter the epigenome [6, 7]. Epigenetics refers to heritable changes in the expression of genes without altering the DNA sequence [8]. The primary epigenetic mechanisms reported to date include DNA methylation, covalent histone modification, and non-coding RNAs (i.e., miRNAs) [9, 10].
Regular physical activity can help to improve overall health and there is increasing evidence showing that these adaptations can result in, or are mediated by, epigenetic modifications [11]. In this context, it has been documented that prescribing physical exercise ensures greater efficacy and better long-term adherence to interventions [12]. However, it is important to differentiate between physical activity and exercise, since both terms are widely used interchangeably. Physical activity is considered as any body movement that increases energy expenditure over the resting level [13], whereas exercise is a subcategory within the area of physical activity, where the activity is planned and structured, with the aim of improving or maintaining physical fitness [14]. Exercise therapy involves the prescription of a specific exercise program that involves performing voluntary muscle contraction and body movements with the aim of alleviating symptoms, improving function, and delaying the deterioration of health [15]. Thus, exercise therapies should include the quantification of variables such as the type of exercise, intensity, duration, frequency, progression, and rest according to the personal needs and individual preferences of each subject, with the objective of maximizing health benefits [12].
In this context, the design of exercise programs should begin with the initial level of physical fitness of each individual considering particularities, such as medical history and assessment of the clinical symptoms [16]. The goal of this review is to provide updated information concerning studies where exercise interventions caused epigenetics modifications related to specific metabolic alterations.
Methods
The present review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Protocols (PRISMA-P) methodology [17]. The results of this review are organized according to specific metabolic alterations related to epigenetic modifications as outcomes of exercise interventions with subsections grouping studies based on healthy, diseased, and trained individuals.
Eligibility Criteria
Studies measuring epigenetic modifications related to metabolic alterations such as obesity, insulin resistance, T2D, inflammation, lipid alterations, cardiovascular risk, and atherosclerosis in response to exercise training interventions or single bouts of exercise were included in this review. Randomized controlled trials, studies without randomization, longitudinal and cross-sectional studies conducted in adult subjects (>18 years) that were published in English were included. After the titles and abstracts were analyzed, reviews, dietary interventions, studies using measurements of other variables or studies focused on other pathologies, were excluded. Duplicated manuscripts were also removed.
Search Strategy and Study Selection
We searched PubMed and EbscoHost Web (Academic Search Complete) databases for the following terms: “Epigenetic and Exercise,” “Epigenetic and Physical Activity,” “Exercise and DNA methylation,” “Exercise and miRNAs,” “Exercise and epigenetic modification,” “Exercise and DNA methylation and Obesity,” “Exercise and epigenetic modification and type 2 diabetes,” “Exercise and DNA methylation and cardiovascular disease,” Exercise and epigenetic and inflammation,” “Exercise and miRNAs and inflammation,” “MicroRNAs/genetics and Exercise/physiology,” “Muscle strength and DNA/genetics,” “DNA methylation/genetics and exercise and muscle skeletal/physiology,” “Exercise/physiology and microRNAs/blood,” “DNA/methylation and genetics and exercise and obesity” that were published between January 2010 and June 2017, finding a total of 2,638 articles. First, we eliminated duplicates and then, after assessing eligibility criteria, we obtained a total of 181 articles. Once studies were read, we excluded those that did not meet the inclusion criteria, resulting in 23 articles that met our criteria. In addition, we included articles from the reference lists of articles (n = 11) previously selected for this review, as long as they met the eligibility criteria so that we could comment on the most relevant papers in the field. Therefore, we included a total of 34 articles for the present analysis. The article titles, abstracts, and keywords provided through the database were evaluated to determine their inclusion (Fig. (Fig.11).
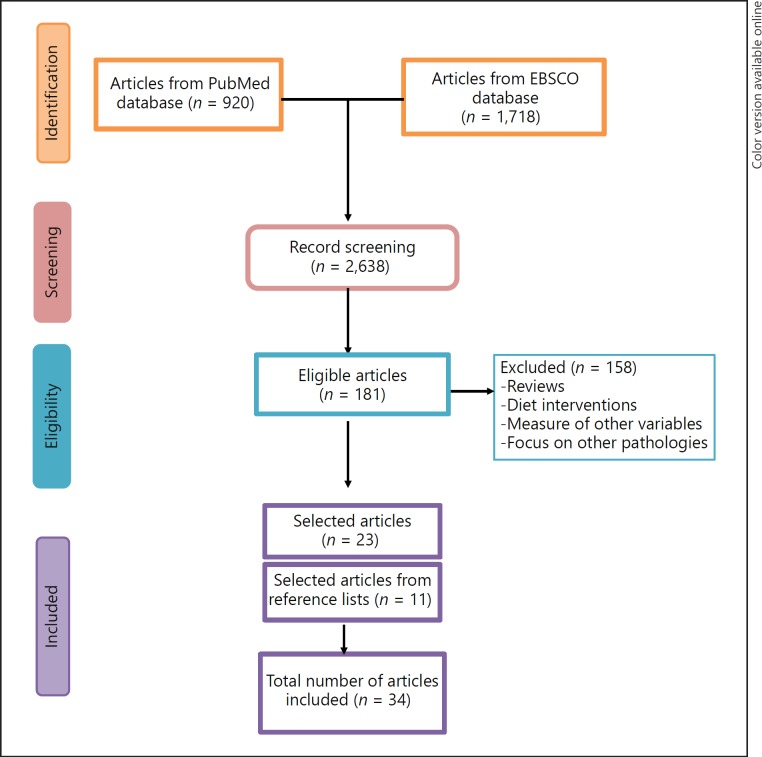
Flow diagram of PRISMA methodology.
Data Extraction and Quality Assessment
The most relevant information about each selected study included in the review was performed using the Population, Intervention, Comparison, Outcome (PICO) format [18]. Data extraction included variables such as type of population, sample size, sex, and age range. Exercise intervention was evaluated based on type and intensity/duration. Additionally, we included type of sample where the epigenetic analysis was performed, outcome measures representing the epigenetic modification evaluated after exercise, and the main results of this modification related to the baseline metabolic alteration. For the quality assessment standard tool of the analyzed studies, we used the Jadad scale for clinical trials [19] and Newcastle-Ottawa for non-randomized studies [20], and the level of evidence was categorized according to the US Preventive Service Task Force report [21].
Results
The studies included in this review show great heterogeneity in terms of sample size, heterogeneity of the characteristics population, exercise intervention (including type, intensity, and duration), and also the different tissues used for the experimental analyses. Therefore, the findings below represent the scientific evidence of epigenetic modifications as outcomes of any exercise interventions related to specific metabolic alterations; however, integrating and interpreting all of this knowledge remains a challenge.
Exercise-Induced Epigenetic Modifications in Genes Related to Insulin Resistance and T2D
Insulin resistance is defined as an impaired ability of cells to respond to insulin action. It usually heralds the onset of T2D, which is characterized by hyperglycemia and occurs when beta cell function is compromised and the pancreas does not produce enough insulin or it does not use it effectively [22]. It has been proposed that a physiological and metabolic adaptation to exercise occurs in skeletal muscle, which is associated with the remodeling of the DNA methylation profile of several genes involved in glucose and lipid metabolism [23]. The data presented below are based on genes related to insulin resistance and T2D, and were grouped according to healthy and diseased individuals.
Healthy Individuals
One of the epigenetic mechanisms that might explain exercise adaptations is the methylation of the PRKAA2 (AMPKα2) gene. AMPKα2 is a catalytic subunit of AMPK and acts as an energy sensor. Indeed, one out of fifteen CpG sites in the PRKAA2gene were altered in whole blood as a result of 4-week rehabilitation training in untrained subjects [24]. Other mechanisms that may be related to DNA methylation in genes involved in retinol metabolism and calcium signaling pathways in skeletal muscle biopsies have been observed after a 6-month endurance training intervention in healthy subjects with a family history of T2D. Since calcium has an important function during exercise through the calcium/calmodulin pathway that improves GLUT-4 translocation and glucose uptake, exercise might reduce the future risk of T2D by epigenetically affecting this mechanism [25].
In addition, it has been found that 60 min of acute exercise (cycling at 65% of peak watt) increased the levels of miR-1 and miR-133a in skeletal muscle biopsies collected from healthy individuals, and after a 12-week training period, subjects improved their VO2max and insulin sensitivity by 19% [26]. In another study, 10 days of endurance training at 75% of VO2max also increased miR-1, miR-133a, and miR-133b levels and reduced miR-9, miR-23a, miR-23b, and miR-31 levels 3 h after exercise. These miRNAs have been associated with the regulation of skeletal muscle regeneration and mitochondrial biogenesis, processes that are crucial for inducing exercise-mediated metabolic adaptations [27]. This favorable adaptation in skeletal muscle metabolism is maintained by regular physical activity; however, 7-day bed rest is enough to suppress these adaptations and the benefits caused by exercise, since rest reduces the levels of oxidative capacity proteins and microRNAs, such as miR-1 and miR-133α. Moreover, these same subjects lose muscle mass and glucose tolerance during this same time frame [28].
A single bout of acute exercise test that included 8 min at 70%, 2 min at 90% of peak heart rate, and then 2 min of rest, in each of the four sessions (a total of 48 min of exercise) was able to increase miR-30 and several miRNAs of the miR-378 family. Although the expression of these miRNAs was not directly associated with insulin sensitivity, several of the miR-378 family members are located in an intronic region of the PPARGC1B gene, whose expression increased after exercise and is associated with mitochondrial function, metabolism, and insulin sensitivity [29].
In another study, authors collected skeletal muscle biopsies from healthy subjects at baseline and 4 h after finishing an acute exercise test at 80% VO2max for 15 min. The mRNA expression analysis showed that the most upregulated gene was the Nuclear Receptor subfamily 4 group A member 3 (NR4A3). Electrical pulse stimulation (EPS) protocol was subsequently used to simulate muscle contraction in C2C12 mouse myotubes cells. After 60 min of EPS, the promoter region of the NR4A3 gene showed intermediate hydroxymethylation followed by hypomethylation, which was accompanied by a subsequent increase in mRNA expression. Since NR4A3 is associated with the maintenance of normal fasting glycemic levels and improved endurance performance, these results suggest that acute exercise might affect metabolic functions through this mechanism [30, 31].
Barrès et al.[32] also demonstrated that acute exercise at 80% of the VO2max induced global DNA hypomethylation, including in the promoter region of some genes involved in T2D (Peroxisome proliferator-activated receptor gamma, coactivator 1α [PPARGC1A],transcription factor A, mitochondrial [TFAM],peroxisome proliferator-activated receptor δ [PPARD],pyruvate dehydrogenase kinase isozyme 4 [PDK4], and citrate synthase[CS]) in muscle. Moreover, the authors performed a dose-response and time-course analysis of DNA methylation after two isocaloric acute exercise trials at either 40% (low-intensity) or 80% (high-intensity) of the VO2max in another cohort. The results showed that the promoter methylation of PPARGC1A, TFAM, MEF2A, PDK4, and PPARD genes was dependent on exercise intensity, being markedly hypomethylated when the subjects performed high-intensity exercise.
Finally, in another study the participant’s upper and lower body strength was assessed through squat, bench press, bench pull, and deadlift exercises by one repetition maximum tests. Subsequently, the participants started a supervised thrice-weekly resistance-training program, which included three sets of 8–12 repetitions of each exercise with a load equivalent to 80% of one repetition maximum tests. At the end of the training program, the genome was altered in 57,384 CpG sites (28,397 hypermethylated and 28,987 hypomethylated) in leukocytes of whole blood in healthy subjects. These differentially methylated CpGs were involved in different biological pathways associated with type 1 diabetes, T2D and calcium signaling [33].
Diseased Subjects
In T2D patients, the DNA methylation and miRNA expression were altered by metabolic plasticity in muscle after 16 weeks of endurance training. These adaptations seem to occur by hypomethylation in the gene bodies of 6-phosphofructo-2-kinase (PFKFB3), histone deacetylase (HDAC4), and the gene encoding the multifunctional Ser/Thr protein kinase (GSK3A) that may regulate glycolytic flux relevant to diabetes rehabilitation [34].
Summary
This above-described set of studies suggests that exercise interventions may have an effect on improving insulin sensitivity via epigenetic modifications in genes and miRNAs related to muscle regeneration, mitochondrial biosynthesis, energy regulation, and calcium signaling pathways. In addition, it seems that epigenetic modifications may depend on the dose/type of exercise; however, more studies need to be performed to confirm these findings. Table Table11 summarizes the outcome of epigenetics modifications related to insulin resistance or T2D in response to exercise training interventions or single tests.
Table 1
Exercise-induced epigenetic modifications related to insulin resistance and diabetes
| Participants | Type of exercise intervention and comparison group | Duration and intensity intervention | Tissue | Outcome measures | Level of evidencea | [Ref.] | |
|---|---|---|---|---|---|---|---|
| epigenetic modification | outcome | ||||||
| Healthy subjects Untrained patients n = 30 (20 F/10 M) Age: 46.1 ± 8.56 y | Rehabilitation training No control group | 5 days per wk for 2 – 3 h during 4 wk | Whole blood | Hypermethylation in one CpG site of AMPKA2 | Improvement of metabolic responses to an energy imbalance | II-1 | [24] |
| Subject with or without FH of T2D n = 28 M FH+ 37.5 ± 4 y FH– 37.5 ± 5.2 y | Endurance training No control group | 1 session of 1-h spinning class and 2 sessions of 1-h aerobic class per wk for 6 months | Skeletal m uscle biopsy | Hypomethylation of genes involved in T2D development, respiratory chain, and calcium signaling pathways | Reduction of future risk of T2D | II-1 | [25] |
| Healthy trained subjects n = 10 M Age: 30.5 ± 5.5 y | Acute exercise test No control group | 60 min of cycle ergometer exercise at 65% of peak watt before and after 12 wk of endurance training | Skeletal m uscle biopsy | Upregulation of miR-1 and miR-133a | Improvement of insulin sensitivity by 19% | II-1 | [26] |
| Healthy subjects n = 9 M Age: 23 ± 5 y | Endurance training No control group | 10 days: 45 min at 75% of VO2 max for 4 days 60 min at 75% of VO2 max one day 90 min at 75% of VO2 max one day 6 × 5 min intervals at 90 – 100% for 4 days | Skeletal m uscle biopsy | Acute endurance exercise: Increase in miR-1, miR-133a, and miR-133b 3h after exercise and reduced miR-9, -23a, -23b and -31 | Increase in transcription of genes involved in muscle regeneration and mitochondrial biogenesis | II-1 | [27] |
| Healthy physically active subjects n = 12 M Age: 26.2 ± 5.3 y | Acute exercise test No control group | 45 min of one-legged knee extensor exercise at 60% of wattmax before and after 7 days of bed rest | Skeletal m uscle biopsy | Bed rest reduced miR-1 and miR-133a content; in addition, reduced HKII, SIRT1, HAD, and CS protein content | Bed rest induced whole body glucose intolerance and decreased muscle metabolic capacity as well as it abolished the exercise-induced adaptive gene response. | II-1 | [28] |
| Healthy subjects n = 14 (9 M/5 F) Age 33 ± 2 y | Acute exercise test No control group | 48 min consisting of 4 sets, each composed of 8 min of cycling at 70%, 2 min at 90% of PHR, and 2 min rest | Skeletal muscle biopsy | Upregulation of miR-30, miR-128, and miR-378 | Alteration of the skeletal muscle gene expression related to insulin sensitivity | II-1 | [29] |
| Healthy subjects n = 10 M Age: 22.6 ± 1.6 y | Acute exercise test No control group | Exercise test at 80% VO 2 max for 15 min | Skeletal muscle biopsy | Increase in hydroxymethylation levels followed by hypomethylation of the <NR4A3 <promoter | Improvement of metabolic function (normoglycemic levels) and endurance capacity | II-1 | [30] |
| Two separate cohorts were used: 1. Healthy sedentary subjects n = 14 Age: 25 ± 1 y 2. Healthy sedentary subjects n = 8 M | First cohorts group: Acute exercise test Second coh orts group: acute exercise test No control group | First cohorts group: Incremental exercise on cycle ergometer until the fatigue Second cohorts group: 2 isocaloric acute exercise trials at 40% and 80% VO2 max on at least 1 week of separate occasions | Skeletal muscle biopsy | First cohort: Intensity-dependent gene hypomethylation Second cohort: Hypomethylation of genes involved in T2D development | Beneficial effect on the prevention and treatment of type 2 diabetes | II-1 | [32] |
| Healthy subjects n = 8 M Age: 21.1 ± 2.2 y | Resistance exercise training No control group | Three sets of 8 – 12 repetitions during 8 wk at 80% of 1RM | Whole blood | Hypermethylation on 28,397 CpG site and hypomethylation on 28,987 | Improvement of diabetes-related signaling pathways | II-1 | [33] |
| Diseased subjects Obesity and T2D patients n = 17 (13 F/5 M) Age: 49 ± 5 y | Resistance exercise training (n = 9) Endurance training (n = 8) | Resistance training group: 2 – 3 sets of 6 – 8 repetitions using machine weights during 16 wk Endurance training group: performed exercise on a cycle ergometer for 40 – 60 min during 16 wk | Skeletal muscle biopsy | Hypomethylation on inflammatory and glucose homeostasis genes | Better regulation of glycolytic flux | I | [34] |
HKII, hexokinase II; SIRT1, sirtuin 1; HAD, 3-hydroxyacyl-CoA dehydrogenase; CS, citrate synthase; 1RM, one repetition maximum; FH, family history; T2D, type 2 diabetes; FH+, subject with family history of T2D; FH–, subject without family history of T2D; M, male; F, female; y, years; PHR, peak heart rate; wk, weeks. a Adapted from US Preventive Services Task Force Guides to Clinical Preventive Services. Agency for Healthcare Research and Quality (US); 1996, where I: represents a randomized clinical trial; II-1: non-randomized controlled trial.
Exercise-Induced Epigenetic Modifications in Pathways Related to Inflammation
Evidence suggests that regular exercise training has the ability to reduce inflammation and its associated complications [35]; however, it is important to understand the mechanisms by which exercise achieves its anti-inflammatory effects. Recent evidence suggests that epigenetic modifications may be involved in the attenuation of the inflammatory response after chronic exercise in both healthy subjects and patients with chronic diseases [33, 36]. However, to improve the inflammatory profile it is important to consider the time, intensity, and type of exercise given that strenuous exercise has been reported to cause harmful inflammation [37, 38].
Trained Subjects
In marathon runners, the levels of ci-miR-134, ci-miR-126, ci-miR-1, ci-miR-133a, ci-miR-499-5p, ci-miR-208a, and ci-miR-146a were found to be significantly upregulated after a 42-km race [39]. These results provide evidence that submaximal and prolonged exercise increases the susceptibility of skeletal muscle damage, cardiac stress, necrosis, and systemic inflammation [39].
Similar results were also found in a study carried out by Gomes et al.[40], which evaluated the levels of circulating miRNAs (ci-miRNAs) before and after a half-marathon race in a group of trained individuals. It was found that miR-1, miR-133a, and miR-206 levels increased after the race. Since the half-marathon is considered a strenuous race that could affect muscle cells, the authors highlighted the potential of these myomiRs as biomarkers of muscle damage, injured, stressed cells, or even adaptation.
In another study, soluble inflammatory mediators and circulating inflammatory miRNAs (c-inflammamiRs) were measured in response to different doses of exercise. It was found that IL-6, IL-8, IL-10, and hs-CRP levels were increased immediately after a marathon and a half-marathon race, except IL-10 in the half-marathon. Furthermore, creatine kinase (CK) levels increased at 0, 24, and 72 h after marathon and half-marathon races compared to baseline levels. CK increases were also observed at 0 and 24 h after a 10-km race. In contrast, the analysis of c-inflammamiRs showed an increase in miR-150-5p immediately after the 10-km race and 12 c-inflammamiRs immediately after the marathon. Thus, the marathon race was associated with an inflammatory profile and increased risk of injury, since an in silico analysis supported an association between the observed c-inflammamiR pattern and pathways of cancer, immune system disorders, and inflammation process. These results suggest that aerobic exercise may have a dose-dependent effect on systemic inflammation [41].
Furthermore, IL-6 levels also increased in a group of trained individuals who performed a treadmill run at 60% of VO2max for 120 min followed by a 5-km time trial. The authors found a correlation between IL-6 protein levels and the methylation of 114 CpG sites in 11 different genes. Although the biological consequence of these methylation changes has not yet been determined, these genes are all involved in inflammatory processes, suggesting a potential role in diseases associated with inflammation [42].
It has also been shown that aging is associated with low-grade inflammation and cell deterioration. Kangas et al.[43] measured changes in serum inflammatory markers in master athletes of different ages in relation to their physical performance. The primary results showed that miR-21 and miR-146a increased and Fas ligand (FasL) decreased with age. On the other hand, athletes performed a 60-m maximal sprint twice, a vertical countermovement jump, knee flexion and bench press. FasL was significantly associated with countermovement jump and bench press, miR-21 and miR-146a with knee flexion and bench press, and miR-146a with sprint performance. The inflammatory markers in serum were modified depending on age range and were associated with low sports performance.
Other authors evaluated a group of highly trained athletes who performed a treadmill test, consisting of running at 80% VO2max for 30 min. Venous blood was collected at four time points during the exercise: before and immediately after exercise, followed by 30 and 60 min of the rest period. Results from this study showed that miRNAs hsa-miR-21-5p, hsa-miR-24-2-5p, hsa-miR-27a-5p, and hsa-miR-181a-5p were upregulated in response to exercise, and downregulated during the recovery period. These miRNAs regulate pathway networks related to immune function, apoptosis, and numerous physiological processes involved in stress adaptation. Thus, this may reflect the self-protective anti-inflammatory response of exercise adaptation [44]. Consistent with this finding, a group of women who performed low-intensity exercise training (tai chi) for at least 3 years showed beneficial epigenetic changes in these age-associated genes. These epigenetic changes might also protect immune competence, and prevent or delay several age-associated diseases [45].
Healthy Subjects
Other authors also observed an upregulation in miR-1, miR-133b, and miR-206, and a concomitant decrease in O2 consumption and increased intracellular calcium concentrations after 12 days of walking training at low-moderate intensity in the mountains. The research group associated the miRNA levels with mitochondrial apoptosis inhibition and attributed these results to an inflammation-associated muscle regeneration response to exercise adaptation [46].
Another study found that interval walking at low intensity at 40% of peak aerobic capacity followed by moderate intensity at 70% of peak aerobic capacity in old men induced hypermethylation of the ASC gene. The hypermethylation in the promoter region of ASC has been associated with lower IL-1β and IL-18 levels, which are associated with T2D, rheumatoid arthritis, and atherosclerosis. Therefore, moderate exercise training may reduce the development of different conditions linked to the systemic inflammation associated with aging and the immune system [47].
Diseased Subjects
In a different study group, patients with chronic obstructive pulmonary disease performed 24 endurance training and peripheral muscle strength sessions, three times per week. The endurance training was taken on a treadmill with 60% of the speed average of the six-minute walking test. The results showed that DNA methylation decreased after the first bout of exercise. Moreover, a negative correlation was found between basal levels of global histone H4 acetylation and IL-4 concentration, and a positive correlation with IL-8 levels. Finally, after 23 training sessions, IL-6 and IL-8 levels were decreased with respect to the first training session [48].
Summary
Findings indicate that prolonged and intense exercise, such as running a marathon or half marathon, is associated with skeletal muscle damage, cardiac stress, and an increase in inflammatory markers that may be mediated through epigenetic modifications in genes related to inflammatory processes and miRNAs associated with skeletal muscle damage. In addition, the inflammatory response induced by exercise appears to depend on the intensity, distance, and time of the exercise. Table Table22 summarizes the outcomes of epigenetic modifications related to inflammation in response to exercise training interventions or single tests.
Table 2
Exercise-induced epigenetic modifications related to inflammation
| Participants | Type of exercise intervention and comparison group | Duration and intensity intervention | Tissue | Outcome measures | Level of evidencea | [Ref.] | |
|---|---|---|---|---|---|---|---|
| epigenetic modification | outcome | ||||||
| Trained subjects Trained runners n = 21 M Age: 51.8 ± 1.4 y | Race distance No control group | Marathon race | Blood s ample (plasma) | Increase in miR-1, miR-126, miR-133a, miR-134, miR-146a, miR-208a, and miR-499-5p | Improvement of skeletal muscle damage, cardiac stress, necrosis, and systemic inflammation | II-3 | [39] |
| Trained runners n = 5 M Age: 31.6 ± 4.39 y | Race distance No control group | Half-marathon race | Blood sample (plasma) | Upregulated of miR-1, miR-133a, and miR-206 | Muscle cell damage or response to stress | II-1 | [40] |
| Amateur trained runners n = 12 M Age: 39.1 ± 2.2 y | Race distance No control group | One endurance race: – 10 km – Half-marathon – Marathon | Blood sample (serum) | Upregulation of miR-150-5p immediately after the 10-km race Upregulation of 12 c-inflammamiRs immediately after the marathon | Association between the c-inflammamiR profile and the inflammatory process | II-3 | [41] |
| Trained subjects n = 8 M Age: 25 ± 4 y | Acute exercise test No control group | 120-min run at 60% VO2 max interspersed with sprints at 90% VO2 max for the last 30 s of every 10 min; followed by a 5-km time trial | Blood sample (PBMCs) | Hypermethylation of 11 genes associated with inflammation pathways | Increased circulation levels of IL-6 and activation of genes involved in inflammation | II-2 | [42] |
| Trained athletes Groups: (A) n = 18 (age: 18 – 39 y) (B) n = 16 (50 – 66 y) (C) n = 18 (66 – 79 y) (D) n = 15 (79 – 90 y) | Resistance exercise test No control group | Maximal 60 m sprint, vertical countermovement jump, knee flexion, and bench press test | Blood sample (serum) | Upregulation of miR-21 and miR-146a | Decrease inflammatory markers in sports performance | III-3 | [43] |
| Trained athletes n = 8 M Age: 21.7 ± 2.6 y | Acute exercise test No control group | Running for 30 min on a treadmill at 80% of VO2 max | Blood sample (serum) | Upregulated miRNAs hsa-miR-21-5p, hsa-miR-24-2-5p, hsamiR-27a-5p, and hsa-miR-181a-5p after exercise | Self-protective anti-inflammatory reaction to exercise | II-1 | [44] |
| Trained subjects n = EXE 237 F n = CON 263 F Age EXE: 64.6 ± 10.4 y Age CON: 62.6 ± 10.9 y | Low-intensity exercise (tai chi) Control: group without exercise or beginners of tai chi | 3 or more years of tai chi practice for at least 1 h per wk | Mouthwash | Hypomethylation of genes associated with age-related processes | Protective effects on the decay of epigenetic functions with age | II-1 | [45] |
| Healthy subjects Healthy subjects n = 7 F Age: 36.3 ± 7.1 y | Low-to-moderate endurance training No control group | 12 days of low-to-moderate walking at low altitude (598 m a.s.l.) The total covered distance was 139,600 m | Skeletal muscle biopsy | Upregulation of miR-1, miR133b and miR206 in samples with decreased O 2 production | Inhibition of the mitochondrial apoptosis pathway and positive correlation with skeletal muscle inflammation | II-1 | [46] |
| Healthy subjects n = 436 (142 M and 294 F) Age EXE: 65.7 ± 7.4 y Age CON: 64.9 ± 7.4 y Age young CON: 19.4 ± 0.9 y | Endurance training Control: group without exercise | Interval walking: 3 min at 40% followed by a 3 min at 70% of peak aerobic capacity for 52 min, 4 times per wk during 6 months | Whole blood | Hypermethylation of; ASC gene in the exercise group | Suppression of excess proinflammatory cytokine expression and inflammation pathway | II-1 | [47] |
| Diseased subjects Participants with COPD n = 13 (7 M and 6 F) Age: 68.5 ± 6.49 y | Endurance training No control group | 24 sessions of 90 min with 60% of the speed average of the 6-min walking test, 3 times per wk | Blood sample (PBMCs) | Global DNA hypomethylation after the first session | After 23 sessions of exercise, a decrease in IL-6 and IL-8 circulation levels | II-1 | [48] |
COPD, chronic obstructive pulmonary disease; PBMCs, peripheral blood mononuclear cells; F, female; M, male; CON, control; EXE, exercise; y, years; wk, weeks. a Adapted from US Preventive Services Task Force Guides to Clinical Preventive Services. Agency for Healthcare Research and Quality (US), 1996, where I represents a randomized clinical trial; II-1 non-randomized controlled trial; II-2 designed cohort studies, cases and controls, multicenter; II-3 studies of multiple series over time with or without intervention as well as uncontrolled experiments.
Exercise-Induced Epigenetic Modifications in Genes Related to Cardiovascular Disease and Blood Lipid Alterations
Exercise training also induces a cardiopulmonary adaptation as well as other cardiovascular changes, including myocardial remodeling [49]and peripheral vascular angiogenesis [50], which reduce the risk of developing some cardiovascular diseases [51] depending on the intensity, type, and duration of the exercise [52].
Trained Subjects
A group of athletes performed an acute exercise test on a cycle ergometer, before and after 90-day of endurance exercise training. Several c-miRNAs involved in angiogenesis and other muscle adaptive processes were measured in plasma in response to acute and chronic exercise. The results showed that peak oxygen consumption increased significantly after 90 days of endurance exercise training. In addition, circulating plasma levels of miR-146a and miR-222 were upregulated after both acute exercise tests (before and after the 90 days of exercise training). In contrast, miR-21 and miR-221 levels were upregulated after the acute exercise test prior to the 90 days of endurance training, and at rest following endurance exercise training, and miR-20a levels were upregulated in response to 90 days of endurance exercise training. The authors also observed a correlation between miR-146a and miR-20a levels and peak oxygen consumption. Thus, these miRNAs suggest a potential marker of cardiorespiratory fitness, peak exercise capacity, and exercise adaptation [53].
In another study, a group of male athletes and a control group were separated according to resistance athletes, endurance athletes, and control subjects of the same age. Blood samples were taken before the exercise tests and at least 12 h after the exercise series, which included tests of handgrip strength, isokinetic dynamometry, pull-ups, vertical jumps, an agility shuttle run, 30 m sprint, a Wingate test, and a continuous ramp-up spiroergometric treadmill test to exhaustion. A significant difference in circulating miR-222 levels was found between male endurance athletes, resistance athletes, and untrained controls of the same age. In addition, plasma levels of miR-21, miR-146a, and miR-221, as well as miR-222, were significantly higher in endurance athletes compared to resistance athletes. These c-miRNAs may be implicated in exercise mode-specific training adaptations [54].
On the other hand, a group of researchers compared the profiles of several miRNAs in healthy male runners before and after marathon race. Immediately after the race, concentrations of miRNAs originating from skeletal muscle (miR-1, miR-133a, miR-499-5p), cardiac tissue (miR-208a), vascular endothelium (miR-126) and related to inflammation (miR-146a) increased significantly with respect to the baseline measurement. However, all these miRNAs were decreased to prerace levels or lower after 24 h of race completion. Additionally, proteins associated with stress and cardiac injury increased after race and remained elevated 24 h after exercise. Notably, miR-1, miR-133a, and miR-208 had a fast regulation 24 h after the marathon. Nevertheless, given the rapid regulation of miR-208, the authors stated that this miRNA could be a sensitive biomarker of the heart response to prolonged exercise [39].
Other authors evaluated 12 young elite athletes who performed a progressive cycle ergometer exercise test for ten 2-min bouts, with a 1-min rest interval between each bout. Blood samples were taken before and immediately after the exercise test, and results showed that 19 miRNAs were altered. An in silico analysis showed that miRNAs altered by exercise could also regulate genes involved in the Jak-STAT, endocytosis and p53 signaling pathways associated with the development of atherosclerotic vascular disease. Thus, acute exercise could have an anti-atherogenic effect through modulation of miRNA levels [55].
The level of physical activity was evaluated in a group of healthy elderly people and showed that those individuals who performed more than one session of light physical activity combined with more than two sessions of heavy physical activity per week had a lower global DNA methylation in blood [56]. This correlation remained statistically significant even when the analyses were adjusted for cardiovascular risk factors (gender, systolic and diastolic blood pressure, fasting glucose, LDL-c and HDL-c, serum triglycerides, current smoking, and BMI). Therefore, it may be concluded that the decrease in physical activity correlates with a worsening of the general state of health and cardiovascular risk [56].
A panel of miRNAs related to muscle and cardiac damage, as well as molecules involved in inflammatory processes, was measured in a group of marathon runners [57]. After the marathon, miR-1, miR-133a, miR-206, miR-208b, and miR-499 levels were all increased. In addition, miR-1, miR-133, and miR-206 showed a correlation with VO2max and running speed at an individual anaerobic lactate threshold, while miR-1 negatively correlated with fractional shortening and miR-133 positively related to the thickness of the intraventricular septum (echocardiographic measurement). No correlations were found between the levels of miRNAs and cardiac proteins [57]. Only two miRNAs showed a moderate discrete correlation with markers of exercise-induced muscle injury (miR-133a with CK and lactate dehydrogenase) and inflammatory markers (miR-206 with CRP and IL-6). This study suggests that miR-1, miR-133a, and miR-206 could be new biomarkers of aerobic exercise capacity due to their high correlation with the evaluated parameters of athletic performance [57].
Another study involved a group of 30 male marathon runners with no cardiovascular history. The group was divided into two age-matched groups, according to the training status: elite runners and non-elite runners. Both groups followed a 10-week training program before a marathon race, where elite runners ran 55 km/week and non-elite runners ran 40 km/week. Before and after the training program, runners performed a cycle ergometer test to evaluate the individual anaerobic threshold and quantify physical fitness. Blood samples were taken before and after the marathon race to measure the levels of circulating plasma miRNAs, and it was observed that the basal levels of miRNAs did not have significant differences between the two groups of runners. Therefore, the 10-week training program did not have an impact on the ci-miRNA levels. However, at the end of the marathon race miR-1, miR-133a, and miR-30a levels were increased significantly. miR-1 and miR-133a were correlated with the increase in left atrial diameter, as well as with CK and MB isoform protein levels. However, these results were only significant in the group of elite runners. Therefore, the expression levels of these miRNAs seem to have a role in cardiac remodeling. These results further suggest that training intensity (elite runners vs. non-elite) may also affect the degree of expression of miRNAs [58].
In another study, 29 well-trained male athletes were included; 15 of which participated in an endurance sport and 14 in a strength sport. The study was performed during a 6-day training period, consisting of two sessions of high physical demand per day and designed for either discipline, except on day 4, where the morning session was not executed. A total of 388 miRNAs were analyzed before and after the training period. In plasma, the miRNA most significantly downregulated was hsa-miR-513b-5p, and the most significantly upregulated was hsa-miR-140-5p, in endurance athletes compared to strength athletes. On the other hand, blood samples showed that hsa-miR-650 was significantly downregulated and hsa-miR-3620-3p was significantly upregulated, in endurance athletes as well. The authors subsequently determined which of the markers showed the best agreement between serum and plasma, and it was found that miR-140-5p had a greater significance in endurance athletes. After evaluating the markers with greater relevance, network analysis showed that the central gene was the vascular endothelial growth factor A (VEGF-A); this gene was targeted by five miRNAs: miR-140-5p (mentioned above), but also miR-378a-3p, miR-361-5p, miR-93-5p, and miR-17-5p. All of these miRNAs were significantly improved in endurance athletes compared to strength athletes. VEGF-A is a key pro-angiogenic regulator of capillary growth in response to physical exercise. Generally, an increase in capillary density is observed only with endurance training, which reflects the requirements of aerobic energy production. However, angiogenesis has also been observed in response to different types of exercise, including endurance and strength training [59].
Healthy Subjects
Healthy subjects who had not previously performed intense exercise underwent a maximum oxygen consumption test before and after a 4-week training program. After the training program, subjects showed improvements in cardiorespiratory fitness and running speed. In addition, total and LDL-c cholesterol levels, resting heart rate, and diastolic blood pressure decreased [60]. A total of 485,577 CpG sites in DNA were evaluated during the study, and the degree of methylation for 81 CpG sites waschanged between 10 and 20% after exercise. Among those that had a major methylation status change (20% of change), 8 sites were hypermethylated and 11 were hypomethylated in regions associated with cardiovascular physiology, including focal adhesion, calcium signaling, and MAPK signaling pathways. Therefore, CpG sites with a greater change in methylation levels after exercise seem to contribute to maintaining cardiovascular health [60]. In contrast, the expression of genes that had the greatest change in DNA methylation as a result of exercise, including miR-21 and miR-210, were measured. Data from pathway analyses suggested that miR-21 is involved in MAPK and Toll-like receptor signaling pathways, apoptosis, and fatty acid metabolism, and its target genes were shown to be associated with diseases like ischemic heart disease, nephropathy, and coronary atherosclerosis, while miR-210 was related to calcium, B-cell receptor, and TGF-beta signaling pathways, and the miR-210 target genes were associated with focal dystonia. Thus, it seems that exercise contributes to methylation changes in genes that are crucial for maintaining good cardiovascular health and improving the serum lipid profile. On the other hand, miR-21 and miR-210 seem to participate in the cardiovascular adaptations related to short-term intense exercise training [60].
Another study that examined epigenetic changes after an acute or endurance exercise intervention was performed in 32 healthy men [61]. Immediately after 60 min of acute exercise (65% of peak watt on a cycle ergometer), the ci-miRNAs miR-106a, miR-221, miR-30b, miR-151-5p, let-7i, miR-146a, miR-652, and miR-151-3p were downregulated in blood. One hour later, miR-338-3p, miR-330-3p, miR-223, miR-139-5p, and miR-143 were upregulated, and 3 h after exercise, miRNA-1 was also overexpressed. After 12 weeks of endurance training program, miR-342-3p, let-7d, miR-766, miR-25, miR-148a, miR-185, and miR-21 were downregulated, while miR-103 and miR-107 were upregulated [61]. There is evidence that some of these miRNAs (e.g., miR-223) are transported by circulating high-density lipoproteins to receptor cells in order to perform their functions [62]. The authors suggest that, considering that exercise modifies miRNA expression, it is possible that lipid transport proteins associated with miRNAS are removed from the circulation after acute exercise [61].
Diseased Subjects
Another group of patients with chronic heart failure performed an exercise training protocol on a bicycle ergometer for 12 weeks. Blood samples were taken before and after training, and HDL-c was isolated by sequential density ultracentrifugation. Subsequently, HDL-c was incubated in human aortic endothelial cells, and it was observed that the expression of miR-126, miR-21, and miR-222 decreased significantly in patients with chronic heart failure compared with healthy controls. The training program was able to significantly improve the HDL-c-induced expression of miR-126 and miR-21 in subjects with chronic heart failure; however, this feature was not observed in healthy subjects. These experiments provide evidence that the exercise program had a suppressive effect on pro-angiogenic miRNAs (miR-126 and miR-21) by decreasing the risk of atherogenesis and endothelial dysfunction [63].
An incremental cardiopulmonary acute exercise test on a bicycle ergometer was performed in a group of patients who had congestive heart failure. Before and immediately after the test, specific miRNAs related to cardiac or muscle physiology (miR-1, miR-133a, miR-133b, miR-499, miR-208a, miR-208b, miR-378, miR-486, and miR-940), angiogenesis (miR-328, miR-126, and miR-221), inflammation (miR-21, miR-146a, and miR-155), and ischemic adaptation (miR-210, miR-21, and miR-146a) were measured in blood, as well as serum biomarkers of muscle and heart damage, and inflammation. After performing the exhaustive acute exercise test, patients showed a significant upregulation of miR-21, miR-378, and miR-940 levels, while no changes were observed in the other miRNAs. The levels of CK, lactate dehydrogenase, and N-terminal pro-brain natriuretic peptide showed an increase after acute exercise, while CK MB isoenzyme (CK-MB), troponin T (Tn-T), and high-sensitive C-reactive protein were not affected. These results indicate that in these patients, the circulating levels of miRNAs may be affected by the duration, intensity, and type of exercise [64].
To demonstrate the effect of both types of exercise, T2D patients were randomized to endurance or resistance training during 16 weeks [34]. The results showed that endurance exercise generated changes in DNA methylation and miRNA expression that were operated by metabolic plasticity in the muscle. Also, an increase in the protein levels of citrate synthase, cytochrome C, short chain-specific acyl-CoA dehydrogenase, mitochondrial 2-oxoglutarate/malate carrier, and GTP:AMP phosphotransferase was observed in the endurance training group but not in the resistance group. After endurance training, hypomethylation was noted in the nuclear respiratory factor 1 (NRF1), fatty acid transporter (SLC27A4), cytochrome P450 (CYP26C1), 6-phosphofructo-2-kinase (PFKFB3), histone deacetylase (HDAC4), and multifunctional Ser/Thr protein kinase (GSK3A) genes; however, the fatty acid synthase (FASN) gene was hypermethylated. After resistance training, hypomethylation of SLC2A4 (GLUT4) and other key genes associated with fatty acid transport and metabolism was observed, suggesting an adaptive response of muscular plasticity as a result of exercise. Twelve genomic regions associated with the cardiovascular system were also hypomethylated in response to endurance exercise. Moreover, the downregulation of miR-29a was linked to the transcriptome network of vascular development. Meanwhile, in response to resistance training, the upregulation of miR-23a and miR-195 was inversely correlated with the expression of genes associated with the development of blood vessels, such as gremlin 1 (GREM1), high mobility group box 2 (HMGB2), and v-raf-1 murine leukemia viral oncogene homolog 1 (RAF1). These changes observed in DNA methylation and miRNA expression in response to chronic exercise, mainly with endurance training, are proposed as a mechanism involved in the improvement and management of metabolic function [34].
Summary
Prolonged and strenuous exercise, such as a marathon race, is not recommended in patients with cardiac alterations, since it has been associated with increased stress and cardiac damage as well as an increase in the inflammatory profile. On the other hand, low-intensity endurance training decreases the risk of developing atherogenesis and endothelial dysfunction through epigenetic modifications in genes associated with lipid and carbohydrate metabolic pathways improving metabolic function and the cardiovascular system. However, these results may be affected by the duration, intensity, and type of exercise, since a single acute exercise test has been associated with increased biomarkers of muscle and heart damage and inflammation-related proteins. Thus, more studies are required to confirm these results. Table Table33 summarizes the outcome of epigenetic modifications related to cardiovascular disease and blood lipid alterations in response to exercise training interventions or single tests.
Table 3
Exercise-induced epigenetic modifications related to cardiovascular disease and blood lipid profile
| Participants | Type of exercise intervention and comparison group | Duration and intensity Intervention | Tissue | Outcome measures | Level of evidencea | [Ref.] | |
|---|---|---|---|---|---|---|---|
| epigenetic modification | outcome | ||||||
| Trained subjects Trained athletes n = 10 M Age: 19.1 ± 0.6 y | Acute exercise test and endurance training No control group | Acute exercise test before and after 90 days of rowing training | Blood samples (plasma) | Upregulation of miR-146a, miR- 222, miR-21, miR-221, and miR- 20a | Improvement of cardiopulmonary fitness and adaptation to exercise | II-3 | [53] |
| Trained athletes Endurance EXE n = 10 (age: 22.6 ± 3.7 y) strength EXE n = 10 (age: 22.2 ± 2.1 y) CON n = 10 (age: 24 ± 2.8 y) | Endurance: race distance Strength: weightlifting and combat sports Control: non-exercise group | All athletes trained for 13 h per wk | Blood sample (plasma) | Endurance exercise increased miR-21, miR-146a, and miR-221, as well as miR-222 compared with strength exercise | These c-miRNAs probably play a role in exercise mode-specific training adaptations | II-1 | [54] |
| Trained runners n = 21 M Age: 51.8 ± 1.4 y | Race distance No control group | Marathon race | Blood sample (plasma) | Upregulation of miR-1, miR- 133a, miR-208a, miR-499-5p, miR-126, and miR-146 | Changes in skeletal muscle damage, cardiac stress and necrosis, and systemic inflammation biomarkers | II-3 | [39] |
| Elite athletes n = 12 M Age: 26 ± 0.6 y | Acute exercise test No control group | Ten 2-min bouts of constant work cycle-ergometer with a 1-min rest interval between each bout at 82% of VO2 max | Blood sample (PBMCs) | After exercise test, 19 miRNAs were altered | Acute exercise could have an anti-atherogenic effect | II-1 | [55] |
| Older trained subjects n = 1,016 Age: 70 y | Endurance training No control group | (a) Light physical activity <2 times a wk and no heavy activity (b) Light physical activity > 1 time a wk and no heavy activity (c) Light physical activity >1 time a wk and heavy activity once or twice a wk (d) Light physical activity >1 time a wk and heavy activity and >2 times a wk | Blood sample (leukocytes) | Hypomethylation of global DNA | It is possible that decreased physical activity is correlated with a worsened health status | II-3 | [56] |
| Trained runners n = 14 M Age: 42.8 ± 6.0 y | Race distance No control group | Marathon race | Blood samples (plasma) | Upregulation of miR-206, miR-1, miR-133a, and miR-206 | Potential role for muscle-and heart-specific miRs in cardiovascular adaptation processes after endurance exercise | II-1 | [57] |
| Trained runners Elite: n = 15 (age: 40.0 ± 1.7 y) Non-elite: n = 15 (age: 40.1 ± 1.4 y) | Endurance training program before a marathon race No control group | For elite athletes 55 km/wk and for non-elite athletes 40 km/wk during 10 wk | Blood sample (plasma) | At the end of the marathon race miR-1, miR-133a, and miR-30a levels were increased significantly | Role in cardiac remodeling | II-1 | [58] |
| Trained athletes Strength EXE n = 14 M Endurance EXE n = 15 M | Resistance and endurance training of high intensity No control group | 6-day training, 2 sessions per day with the exception of day 4, which was the only one session | Blood sample (plasma) | Endurance training upregulation of miR-140-5p, miR -378a-3p, miR-361-5p, miR-93-5p, and miR-17-5p | After evaluating the markers with greater relevance, network analysis showed that the central gene was VEGF-A | II-1 | [59] |
| Healthy subjects Healthy subjects n = 19 M Age: 21.1 ± 2.7 y | Sprint interval training No control group | 20 training sessions, 3/wk during 4 wk | Whole blood | Hypomethylation of genes involved in cardiovascular disease and upregulation of miR-21 and miR-210 (influence cardiovascular physiology) | Improvement of cardiorespiratory fitness | II-1 | [60] |
| Healthy subjects Acute EXE n = 13 M (age: 28 ± 8 y) Endurance EXE n = 7 (age: 28 ± 5 y) | Acute exercise test Endurance exercise training | Acute exercise: 60 min on a cycle ergometer at 65% of peak watt Endurance training: 5 times per wk during 12 wk on a cycle ergometer | Blood samples (plasma) | Acute training: Immediately after 60 min of exercise: downregulation of 8 ci-miRNAs 1 h after exercise: upregulation of 5 miRNAs 2h after exercise: upregulation of 1 miRNA After endurance training: Downregulation of 7 ci-miRNAs and upregulation of two ci-miRNAs | It is possible that lipid transport proteins associated with miRNAs are eliminated from the circulation after acute exercise | II-1 | [61] |
| Diseased subjects Patients with CHF n = 8 (age: 63 ± 3 y) CON n = 8 (age: 67 ± 4 y) | Endurance training Control: healthy subjects trained on bicycle ergometer at 65 – 75% of VO2max | During the first 3 wk, 3 – 6 times daily for 5 – 20 min at 50% of VO2 max, then 20 – 30 min/day at 60% of VO2 max for 12 wk Control: 4 × 30 min/day, 5 times per wk during 4 wk | Blood sample (serum) | Improvement of HDL-induced miR-126 and miR-21 expression in CHF patient | Reduced risk of atherogenesis and endothelial dysfunction | II-2 | [63] |
| Heart failure participants n = 28 M Age: 59.07 ± 1.79 y | Acute exercise test No control group | Starting from 60 rpm for 3 min without any resistance, and continued pedaling at 60 rpm throughout the test, until exhaustion | Blood sample (serum) | Upregulation of miR-21, miR-378, and miR-940 | Increase in CK, LDH, and NT-proBNP levels after acute exercise | II-1 | [64] |
| Obesity and T2D patients n = 17 (13 F/5 M) Age: 49 ± 5 y | Resistance exercise training (n = 9) Endurance training (n = 8) | Resistance training group: 2 – 3 sets of 6 – 8 repetitions using machine weights during 16 wk Endurance training group: performed exercise on a cycle ergometer for 40 – 60 min during 16 wk | Skeletal muscle biopsy | Endurance training: Downregulation of miR-29 a Resistance training: upregulation of miR-23a and miR-195 | Changes in the expression of IGF2, CD34, MAP2K3, and VEGFB proteins associated with blood vessel development | I | [34] |
CK, creatine kinase; LDH, lactate dehydrogenase; NT-proBNP, N-terminal pro-brain natriuretic peptide; IGF2, insulin-like growth factor 2; CD34, transmembrane phosphoglycoprotein protein encoded by the CD34 gene in humans; MAP2K3, mitogen-activated protein kinase kinase 3; VEGFB, vascular endothelial growth factor B; CHF, chronic heart failure; T2D, type 2 diabetes; M, male; F, female; y, years; EXE, exercise; wk, weeks. a Adapted from US Preventive Services Task Force Guides to Clinical Preventive Services. Agency for Healthcare Research and Quality (US), 1996, where I represents a randomized clinical trial; II-1 non-randomized controlled trial; II-2 designed cohort studies, cases and controls, multicenter; II-3 studies of multiple series over time with or without intervention as well as uncontrolled experiments.
Exercise-Induced Epigenetic Modifications in Genes Related to Obesity
A sedentary lifestyle in obese individuals contributes to the pathophysiological changes in skeletal muscle, mainly in mitochondrial density, oxidative enzymes, and insulin sensitivity [65]. In turn, it is known that insulin resistance in skeletal muscle reduces lipid oxidation, increases available intramuscular triglycerides, lipid metabolites, and oxidative stress [9, 66]. Evidence suggests that regular physical exercise benefits obesity and its comorbidities, such as insulin resistance by epigenetic mechanisms [67, 68, 69].
Healthy Subjects
One of the most studied genes is PPARGC1A, since it has a major role in mitochondrial biogenesis [67]. Muscle biopsies from 11 non-obese, normoglycemic subjects were obtained before and after performing an acute exercise test on a cycle ergometer [67]. The results showed a loss of nucleosome positioning surrounding the −260 nt region, a known site for the regulation of DNA methylation. After exercise, a positive correlation was found between changes in PPARGC1A mRNA expression and the nucleosome repositioning in the site of regulation of DNA methylation in muscle cells. When subjects were categorized into high and low responders, depending on the methylation status that was observed after the exercise, it was found that high responders significantly decreased intramyocellular lipids compared with low responders. These results were compared to myotubes isolated from muscle biopsies obtained from subjects with obesity and T2D that were treated with a palmitate, forskolin, and ionomycin cocktail to mimic the effect of exercise. Interestingly, the results also showed a decrease in nucleosome occupancy surrounding the −260 nt region [67].
Exercise-induced epigenetic changes have also been studied in the adipose tissue of 31 sedentary healthy subjects (15 with and 16 without a family history of T2D) who performed endurance exercise training for 6 months. After the exercise program, increases in DNA methylation in 16,470 CpG sites and decreases in 1,505 CpG sites were reported. From them, 1,009 CpG sites changed more than 5% (911 hypermethylated and 98 hypomethylated) in response to exercise. Of the genes that showed significant changes after the 6-month exercise program, 24 CpG sites were found to be located in 18 candidate genes for obesity and 45 CpG sites were located in 21 candidate genes for T2D. It should be emphasized that 10 of these sites were mapped to the KCNQ1 gene and 6 sites to the TCF7L2 gene. Finally, changes in expression levels were observed in four of these candidate genes (HHEX, IGF2BP2, JAZF1, and TCF7L2), where higher levels of DNA methylation were associated with lower levels of mRNA expression. To better understand the in vivo methylation changes of adipose tissue caused by exercise, KCNQ1 and TCF7L2 genes were silenced in 3T3-L1 adipocytes. An in vitro experiment observed that the downregulation of the expression levels of these two genes increased lipogenesis in both basal and insulin-stimulated states. Exercise alters DNA methylation in subcutaneous adipose tissue, probably affecting lipogenesis and other metabolic processes in adipocytes [70].
Diseased Subjects
As mentioned above, Rowlands et al. [34] also measured the methylation changes in genes involved in lipid metabolism in obese and T2D sedentary subjects. The participants performed resistance or endurance training for 16 weeks, and skeletal muscle biopsies were taken before and after the intervention. Results showed that both resistance and endurance training led to hypomethylated DNA. In the endurance-training group, the best represented functional networks were those related to lipid metabolism, carbohydrate metabolism, metabolic disease, and cardiovascular function. The miRNA-mRNA regulation analysis highlighted miR-29a and miR-132 in endurance training, and miR-1207-5p and miR-195 in resistance training, which regulate genes involved in the transcriptional regulation, lipid and glucose metabolism, and blood vessel development, respectively. Moreover, hypomethylation of NRF1 and hypermethylation of FASN were found following endurance training, which was associated with a decrease in metabolic and physiological lipids. On the other hand, resistance training increased the activity of mitochondrial oxidative and respiratory enzymes. Since SLC2A4 and genes responsible for the transport and metabolism of fatty acids (ACSL1, LRP10, and SLC27A1) were hypomethylated, a possible role for DNA methylation in the skeletal muscle plasticity generated by this type of training was hypothesized. These changes were observed in the accumulation of intracellular lipids in response to chronic endurance training. Particularly, the hypermethylation of FASN following this type of exercise contributed to the decrease in fatty acid synthesis and lipid accumulation, which was in accordance with the underexpression of the transcription factor sterol regulatory element-1 (SREBP1c), which regulates the activity of FASN in skeletal muscle. These changes in intramuscular lipid content of subjects with T2D suggest a better glucose uptake and utilization in skeletal muscle [34].
Other studies evaluated plasma miRNAs in 33 obese elderly adults who walked 4 days a week for 5 months. The main results showed that 4 miRNAs were significantly altered after the exercise program: miR-376a-5p increased, while miR-16-5p, miR-27a-3p, and miR-28-3p all decreased. In addition, 800 miRNAs were characterized and could serve as gait speed indicators in the adaptations to exercise in this type of patients [71].
Summary
Chronic endurance training may decrease the intracellular lipid accumulation through epigenetic modifications in genes associated with the synthesis and accumulation of fatty acids. Also, modifications have been observed in some networks related to carbohydrate metabolism, metabolic diseases, cardiovascular function, and in the pathophysiology of obesity and T2D affecting lipogenesis and other metabolic processes in adipocytes. When training was combined with resistance exercise sessions, an increase in the development of blood vessels and other epigenetic modifications related to a decrease in circulating lipid levels was observed. Nevertheless, more studies are needed that combine endurance and resistance exercise programs to demonstrate that both types of exercise are necessary to experience the physiological and metabolic adaptations related to epigenetic modifications. Table Table44 summarizes the outcome of epigenetic modifications related to obesity in response to exercise training interventions or single tests.
Table 4
Exercise-induced epigenetic modifications related to obesity and lipid metabolism
| Participants | Type of exercise intervention and comparison group | Duration and intensity intervention | Tissue | Outcome measures | Level of evidencea | [Ref] | |
|---|---|---|---|---|---|---|---|
| epigenetic modification | outcome | ||||||
| Healthy subjects Healthy subjects n = 11 M Age: 24 ± 1 y | Acute exercise test No control group | Exercise test on cycle ergometer at 50% VO2max until expenditure of 650 kcal | Skeletal muscle biopsy | Loss of nucleosome positioning at (− 260 nt), a site of regulation of DNA methylation of the ; PGC1 α gene | Reduction of intramyocellular lipid content | II-1 | [67] |
| Healthy subjects n = 31 FH+ n = 15 M FH− n = 16 M Age: 37.3 ± 4.4 y | Endurance training Control: subject without family history of T2D | 1 session of 1 h spinning and 2 sessions of 1 h aerobics for 6 months with an average of 42.8 ± 4.5 sessions | Adipose tissue biopsy | Changes in global DNA methylation and methylation changes in 18 obesity candidate genes and 21 T2D genes in adipose tissue | Alteration of DNA methylation levels in genes involved in lipogenesis and lipid metabolism in adipocytes | I | [70] |
| Diseased subjects Obesity and T2D patients n = 17 (13 F/5 M) Age: 49 ± 5 y | Resistance training (n=9) Endurance training (n=8) | Resistance training group: 2 – 3 sets of 6 – 8 repetitions using machine weights during 16 wk Endurance training group: performed exercise on a cycle ergometer for 40 – 60 min during 16 wk | Skeletal muscle biopsy | Endurance training: Hypomethylation of the NRF1 promoter region and hypermethylation of; FASN promoter region | Decrease of fatty acid synthesis and intracellular lipid accumulation | II-1 | [34] |
| Obese subjects n = 33 Age: 69.3 ± 3.6 (24 M/9 F) | Endurance training No control group | 15 – 20 min walking at 50% HRR during the 1st wk and 30 min at 65 – 70% HRR the rest of the intervention (4 days a wk for 5 months) | Blood sample (plasma) | miR-376a-5p increased, while miR-16-5p, miR-27a-3p, and miR-28-3p decreased | Adaptations to exercise | II-1 | [71] |
ci-miRNA, circulating microRNA; NRF1, nuclear respiratory factor 1; FASN, fatty-acid synthase; T2D, type 2 diabetes; FH, family history; HRR, heart rate reserve; wk, weeks. a Adapted from US Preventive Services Task Force Guides to Clinical Preventive Services. Agency for Healthcare Research and Quality (US), 1996, where I represents a randomized clinical trial; II-1 non-randomized controlled trial.
Conclusion
Physical inactivity and sedentary behavior are leading risk factors for the expansion of global health morbidities and mortality [72], being related to the development of metabolic alterations that include insulin resistance, T2D, inflammation, cardiovascular risk, obesity, cancer, and dyslipidemias [73]. Evidence suggests that regular physical exercise can improve the negative consequences of physical inactivity via epigenetic alterations in different tissues, such as adipose, skeletal muscle, and blood cells (Fig. (Fig.2)2) [74].
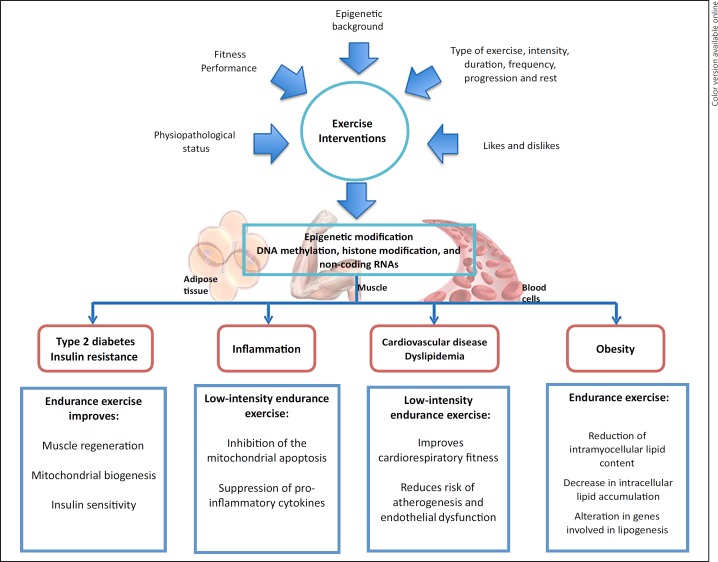
Epigenetic modifications related to exercise interventions.
The available evidence regarding epigenetic modifications as outcomes of endurance exercise interventions seems to implicate biomarkers related to muscle regeneration, mitochondrial biosynthesis, energy regulation, and calcium signaling pathways. On the other hand, prolonged and intense exercise, such as a marathon or half marathon, is associated with skeletal muscle damage, cardiac stress, and an increase in inflammatory markers. Thus, these types of distance races should not be recommended to patients with diagnosed cardiac impairments.
In contrast, endurance training that consists of low-to-moderate intensity exercises, such as tai chi, walking intervals, or very-low-intensity race decreases the risk of developing atherogenesis and endothelial dysfunction, as well as improves metabolic function, the cardiovascular system, and inflammatory profiles through the inhibition of mitochondrial apoptosis and muscle regeneration. Further, it seems that regular endurance training may also decrease the risk of developing obesity and T2D through affected lipogenesis and other metabolic processes in adipocytes.
The studies summarized in this review show that exercise interventions can alter the epigenome, and that these outcomes could be related to specific metabolic pathways. However, more studies are needed to demonstrate more consistent results because there are several limitations in the studies reported to date, including small sample size, heterogeneous populations, the different duration of exercise interventions, the variety of exercise tests available, and the different epigenetic modifications measured in different tissues. Thus, given the heterogeneity and complexity of the existing literature, it is currently not possible to propose a specific recommendation about the type, intensity, or duration of exercise that could be beneficial for different subsets of the population (healthy, diseased, and/or trained). Nevertheless, this review does highlight the importance of exercise for health and shows the need to perform more research in this emerging area to identify epigenetic biomarkers that could serve as indicators of exercise adaptations.
Content retrieved from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6921698/.